Cardiometabolic Disease and the Gut Microbiome
Published on: 3 May 2023
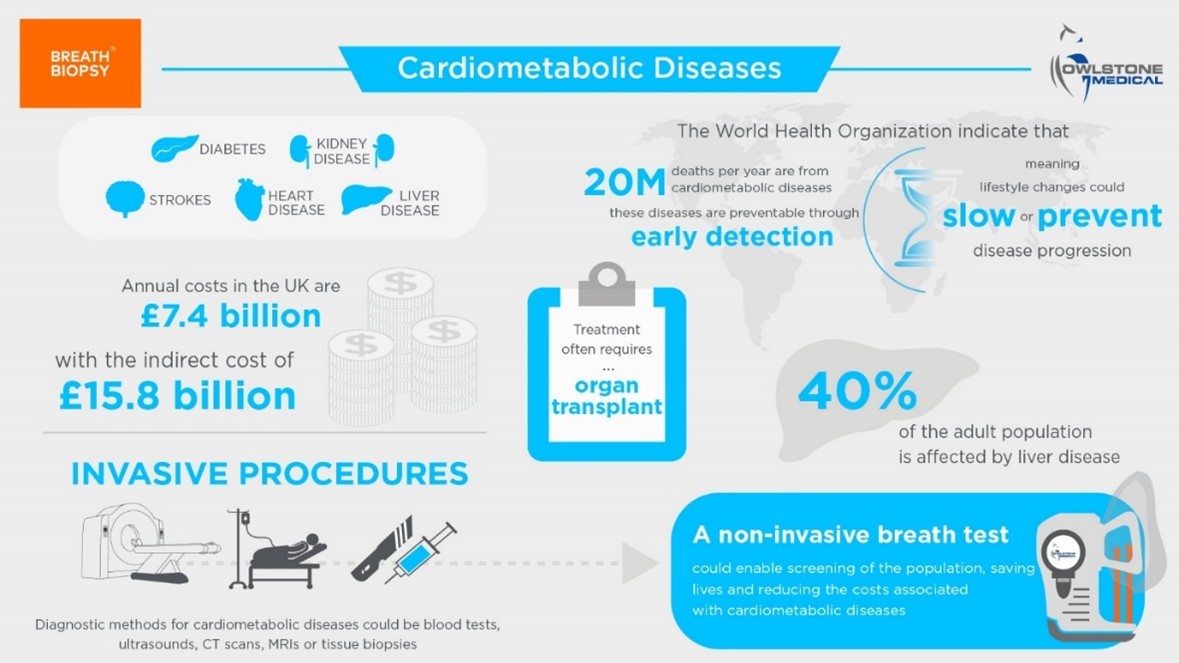
Cardiovascular and cardiometabolic diseases (CVMD) refer to a group of conditions affecting the heart, blood vessels, and metabolism, including diabetes, strokes, heart, liver, and kidney diseases. The etiology of CVMD is complex and multiple biological, environmental, and social factors are implicated. Known risk factors for the development of CVMD include family history, dietary patterns high in sugar, salt, and processed foods, inactivity, and living with overweight and obesity (1). CVMD have a substantial worldwide disease burden, accounting for over 393 million disability-adjusted life years (DALYs, lost years of healthy life) and 45% of all non-communicable disease deaths in Europe (2,3). Given the impact of CVMD, understanding their underlying mechanisms and interactions with other bodily systems are imperative.
 Could the gut microbiome be involved in CVMD?
Could the gut microbiome be involved in CVMD?
Although the gut microbiome has many beneficial roles in food breakdown, nutrient metabolism, and bioactive molecule production, microbial compositional changes have been associated with the development and progression of disease, including CVMD. For instance, such changes have been reported in patients with risk factors for CVMD, such as hypertension and insulin resistance (4). However, a key consideration when assessing the potential impact of compositional changes is their associative nature – this introduces a challenge in understanding the causative mechanisms of disease. It is often unclear whether changes in the gut microbiome drive CVMD disease states, or if living with CVMD leads to alterations in the gut microbiome (4). This challenge is compounded by the prevailing use of sequencing techniques that often fail to reach strain level resolution, making it difficult to identify less abundant microbes that could be implicated in the etiology of CVMD (4).
A growing body of literature highlights the potential mechanistic relationship between microbially-produced bioactive molecules and the development of CVMD. In this blog, we’ll discuss several microbially-derived molecules that may be implicated in CVMD pathophysiology and highlight how novel approaches, such as exploring the composition of exhaled breath, can support research into the relationship between CVMD and the gut microbiome.
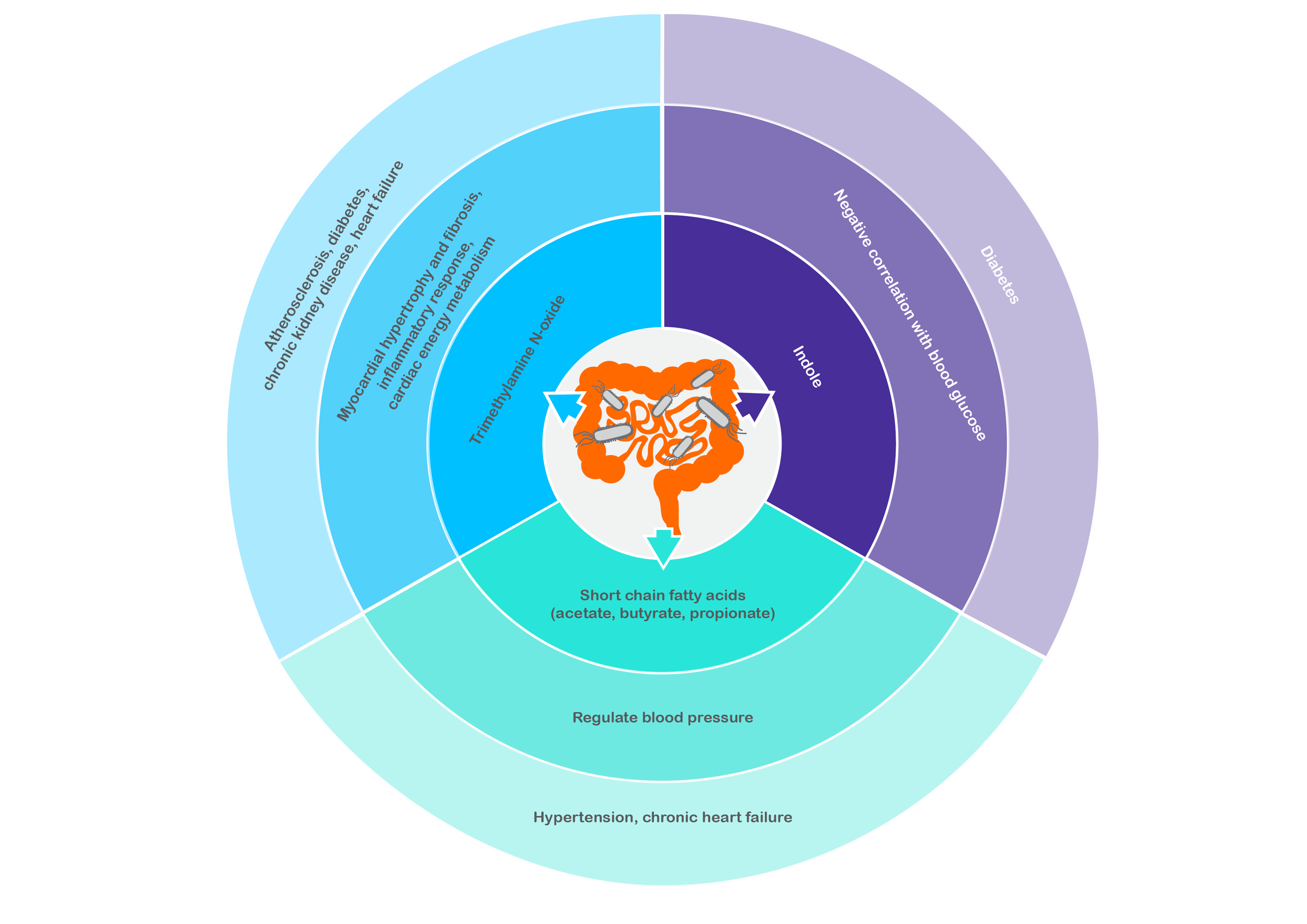
Trimethylamine N-oxide
Dietary components from animal-derived products (e.g., phosphatidylcholine, choline, L-carnitine) are metabolized by bacteria in the gut to trimethylamine (TMA), which is subsequently converted to trimethylamine N-oxide (TMAO) in the liver. Higher levels of serum TMAO have been associated with an increased risk of atherosclerosis (5), diabetes (6), and chronic kidney disease (7), highlighting the potential negative effects of interactions between bacterial and human metabolism.
Studies have found the gut microbial composition between healthy controls and patients with chronic heart failure to be significantly different. Key differential characteristics include a decrease of Faecalibacterium prausnitzii and an increase of Ruminococcus gnavus (8). Metagenomic analyses also highlighted an elevation in microbial genes associated with TMAO production in the gut microbiome of heart failure patients (8).
Additionally, a large study of 720 heart failure patients reported that higher plasma TMAO levels were associated with a 3.4-fold increase in mortality risk and remained predictive of five-year mortality risk after consideration of traditional risk factors such as age, blood pressure, smoking status, and diabetes (9). Experimental studies exploring the physiological mechanisms of TMAO in heart failure have identified that TMAO promotes myocardial hypertrophy and fibrosis, induces inflammatory responses, and impacts cardiac energy metabolism, all of which can contribute to the development of heart failure (10). The axis between the gut microbiome, TMAO, and heart failure holds promise as a therapeutic target for heart failure treatment. Many approaches involving decreasing TMAO levels by manipulating the gut microbiota to produce less TMA are under investigation, including dietary, probiotic, prebiotic, and phytochemical-based therapies (10).
Short-chain fatty acids
When dietary fiber is fermented by gut bacteria, short-chain fatty acids (SCFAs) such as butyrate, acetate, and propionate are produced, absorbed into systemic circulation, and are involved in host processes including glucose homeostasis, lipid metabolism, and neurogenesis. Through their binding with G-protein-coupled receptors, SCFAs are thought to regulate blood pressure, a factor implicated in CVMD development. SCFAs can have hypertensive (increasing blood pressure) or hypotensive (lowering blood pressure) effects depending on the receptors to which they bind (11).
Lower levels of serum acetate and propionate have been reported in patients with hypertension (12). Exploring the gut microbiome of patients with pulmonary arterial hypertension revealed a less diverse microbiome and fewer copies of microbial genes responsible producing anti-inflammatory SCFAs, as well as lower levels of plasma SCFAs compared to healthy control subjects (13). Long-term blood pressure dysregulation forces the heart to work harder and can increase the risk of heart attack, failure, and sudden cardiac death. Interestingly, the presence of bacteria involved in SCFA metabolism is also reportedly reduced in chronic heart failure patients (8), highlighting the interrelated nature of different CVMDs and the gut microbiome.
Indole
The relationships between CVMD and molecules like TMAO and SCFAs are relatively well-established, but other molecules might be able to offer novel perspectives. Indole, a bacterial metabolite of tryptophan, has recently been identified as a new tentative biomarker of blood glucose in diabetic patients (14). A negative correlation between exhaled indole (tentatively assigned to the mass peak at m/z 118.006) and blood glucose was observed when breath samples were collected for six hours after type 1 and 2 diabetes patients ingested a standardized meal (14). The metabolic capacity of bacteria from the genera Bacteroides, Clostridium, and Escherichia to produce indole from tryptophan suggests that exploring their relative abundance in patients with diabetes may offer novel perspectives on the diabetic gut microbiome and its association with disease.
How can Owlstone Medical support you with your CVMD research?
Although molecules such as TMAO, SCFAs, and indole can be measured in fecal samples and blood components, non-invasive methods such as breath sampling may be able to offer unique perspectives into their relationships with CVMD. An additional benefit of breath is that it offers a dynamic, real-time insight into the inner workings of the gut microbiome – something fecal analysis cannot provide since whole gut transit time can range from 10 to 73 hours (15). Both indole and several SCFAs (such as acetic, butyric, and propionic acid) are present on breath, as is TMA, the microbially-produced precursor of TMAO. These compounds have been identified in our Breath Biopsy® VOC Atlas – a catalog of identified volatile organic compounds (VOCs) that are commonly found on breath in a heterogenous, healthy population.
You can learn more about our work in the CVMD field here, or get in touch to discuss how our Breath Biopsy® platform could support your research into the gut microbiome and CVMD.
References
- Casas R, Castro-Barquero S, Estruch R, Sacanella E. Nutrition and Cardiovascular Health. Int J Mol Sci. 2018;19(12). doi:10.3390/IJMS19123988
- Roser M, Ritchie H, Spooner F. Burden of Disease. OurWorldInData.org. Published 2021. Accessed May 4, 2023. https://ourworldindata.org/burden-of-disease#citation
- Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232-3245. doi:10.1093/EURHEARTJ/EHW334
- Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553. doi:10.1161/CIRCRESAHA.120.316242
- Randrianarisoa E, Lehn-Stefan A, Wang X, et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with early Atherosclerosis in Humans. Scientific Reports 2016 6:1. 2016;6(1):1-9. doi:10.1038/srep26745
- Dambrova M, Latkovskis G, Kuka J, et al. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Experimental and Clinical Endocrinology and Diabetes. 2016;124(4):251-256. doi:10.1055/S-0035-1569330/ID/R11-2015-0457-DIA-0031
- Zhang W, Miikeda A, Zuckerman J, et al. Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice. Scientific Reports 2021 11:1. 2021;11(1):1-11. doi:10.1038/s41598-020-80063-0
- Cui X, Ye L, Li J, et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci Rep. 2018;8(1). doi:10.1038/S41598-017-18756-2
- Tang WHW, Wang Z, Fan Y, et al. Prognostic Value of Elevated Levels of Intestinal Microbe-Generated Metabolite Trimethylamine-N-oxide in Patients with Heart Failure: Refining the Gut Hypothesis. J Am Coll Cardiol. 2014;64(18):1908. doi:10.1016/J.JACC.2014.02.617
- Zhang Y, Wang Y, Ke B, Du J. TMAO: how gut microbiota contributes to heart failure. Translational Research. 2021;228:109-125. doi:10.1016/J.TRSL.2020.08.007
- Tokarek J, Budny E, Saar M, et al. Does the Composition of Gut Microbiota Affect Hypertension? Molecular Mechanisms Involved in Increasing Blood Pressure. Int J Mol Sci. 2023;24(2). doi:10.3390/IJMS24021377
- Ward NC, Carnagarin R, Nolde JM, et al. Circulating short-chain fatty acids in hypertension: a reflection of various hypertensive phenotypes. J Hypertens. 2022;40(8):1589-1596. doi:10.1097/HJH.0000000000003190
- Moutsoglou DM, Tatah J, Prisco SZ, et al. Pulmonary Arterial Hypertension Patients Have a Proinflammatory Gut Microbiome and Altered Circulating Microbial Metabolites. Am J Respir Crit Care Med. 2023;207(6):740-756. doi:10.1164/RCCM.202203-0490OC
- Fink H, Maihöfer T, Bender J, Schulat J. Indole as a new tentative marker in exhaled breath for non-invasive blood glucose monitoring of diabetic subjects. J Breath Res. 2022;16(2). doi:10.1088/1752-7163/AC4610
- Lee YY, Erdogan A, Rao SSC. How to assess regional and whole gut transit time with wireless motility capsule. J Neurogastroenterol Motil. 2014;20(2):265-270. doi:10.5056/JNM.2014.20.2.265