
EVOLUTION
The development of a breath test for the early detection of lung cancer
INTRODUCTION
Lung Cancer
Lung cancer is one of the most common forms of cancer in the world with 2.2 million new cases in 2020 and is a leading cause of death (1.8 million globally in 2020), with 5-year survival at 25% in the United States and just 15% in the United Kingdom (1–3). The best way to increase the current poor survival probabilities for patients with lung cancer is to detect the disease early when it can be treated with curative intent by surgery or radiotherapy: diagnosis at an early stage increases the 5-year survival rate to 56%, compared to just 5% for the late stage (4). In the United States there are guidelines in place for using Low-dose CT (LDCT), and a similar program is being launched in the U.K. to screen for lung cancer. This use of LDCT for lung cancer screening has been shown to detect more cancers at an early stage, however, uptake is less than 6% due to inconvenience, radiation dose, test performance, and in some cases, cost.
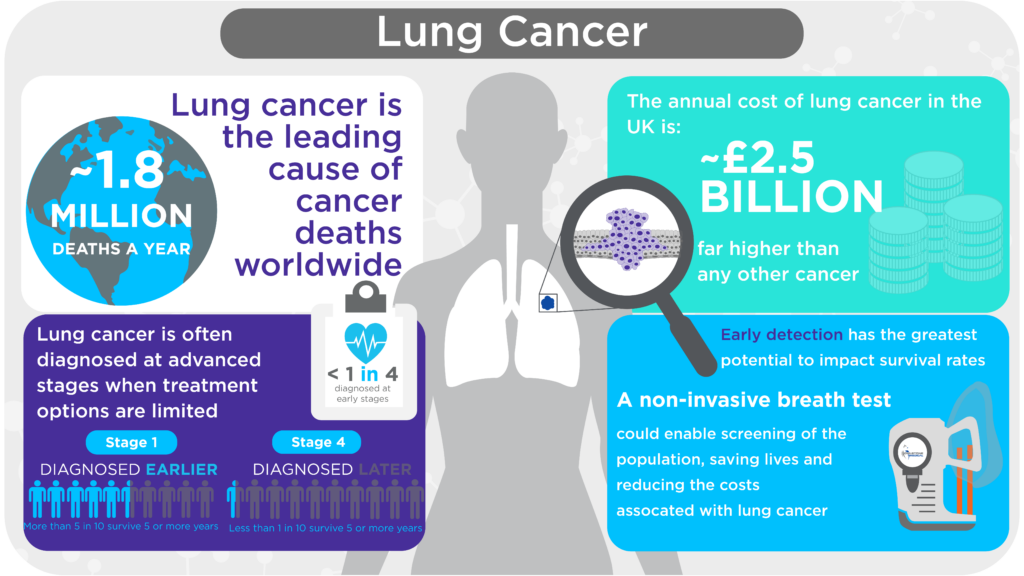
Therefore, there is a significant need for alternate screening tools for lung cancer. Breath analysis has emerged as a highly promising, non-invasive, potential solution. An effective breath test for lung cancer could further increase the efficacy, reach, and accessibility of lung cancer screening.
Breath contains volatile organic compounds (VOCs) that can inform on underlying physiology, therefore could offer a much-needed alternative approach to overcome the current barriers to lung cancer screening (5). For a lung cancer test for screening and early detection to be successful, it must be able to detect signals coming from even small early-stage tumors, alongside potential multiple co-morbidities. Through careful evaluation of the metabolic pathways underpinning the production of the volatile compounds linked to the presence of cancer, the EVOC® probe approach was suggested to be able to improve the signal-to-noise ratio of breath tests and has shown success for a candidate test to diagnose liver disease (6,7).
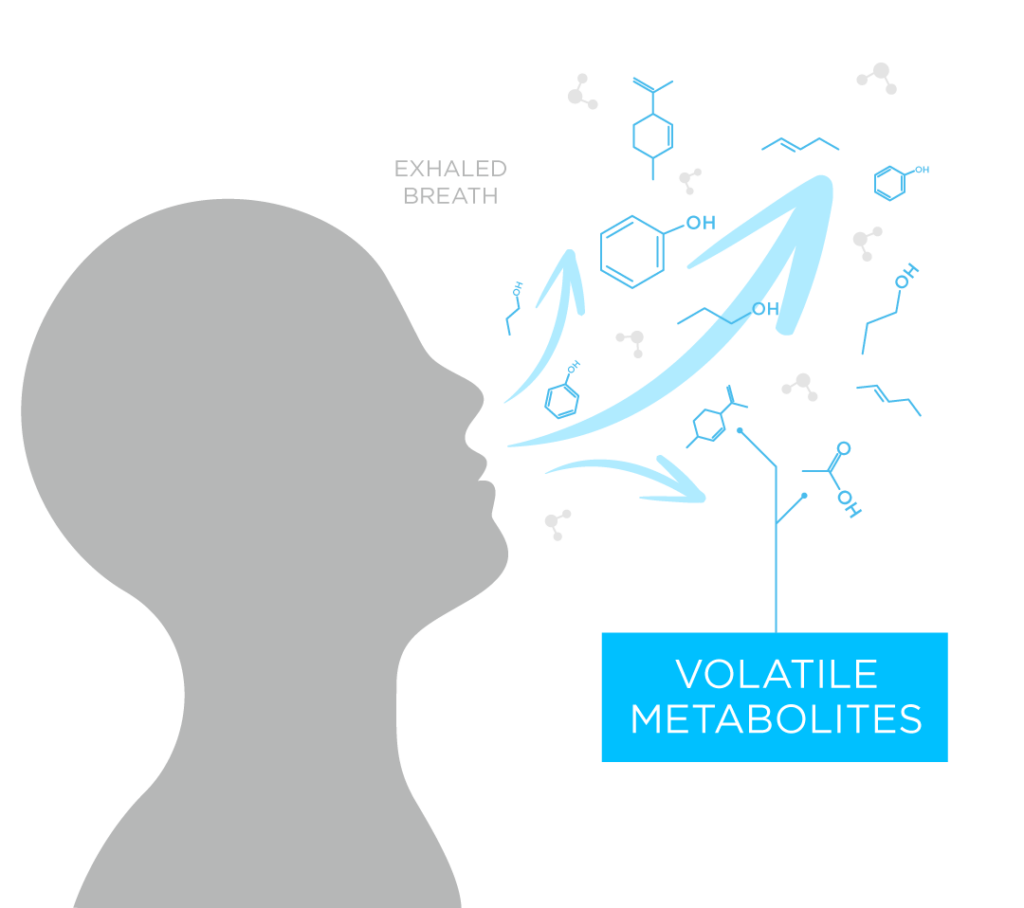
The D5-EthGlu Probe
A Probe-Based Breath Test for Lung Cancer
An innovative and alternative approach for breath-based testing involves the utilization of exogenously produced volatile compounds as probes. Owlstone Medical has developed Exogenous Volatile Organic Compound (EVOC®) Probes. These are compounds that can be introduced into the body (through ingestion or inhalation) to explore how the probe is absorbed, metabolized, and excreted through dynamic analysis of the changes in breath VOC composition in response. This approach can target specific pathways of interest and amplify the signal from these pathways for better breath test performance. This concept draws similarities to how labeling urea with 13C is currently employed as a metabolic probe in clinical settings to evaluate H. pylori infection within the gastrointestinal tract.
In the EVOLUTION trial, the diagnostic potential of D5-ethyl-βD-glucuronide (also known as D5-EthGlu or OWL-EVO1) as an exogenous volatile organic compound (EVOC®) probe for detection of lung cancer is currently being evaluated (8,9).
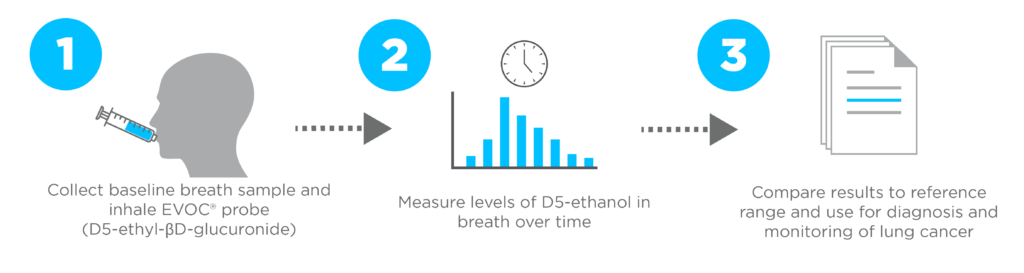
EVOLUTION
The EVOLUTION Trial
Under normal conditions, the enzyme β-glucuronidase is present within cells in the lysosome and is responsible for the catalytic hydrolysis of β-d-glucuronides. Research in humans and mice has pointed towards β-glucuronidase being differentially expressed in the tumor microenvironment (includes tumor cells and the extracellular space with surrounding cells, blood vessels and tissues) (10,11) of many types of lung cancer, including squamous, adeno, and adeno-squamous non-small-cell lung cancers (12). Interestingly, high levels of β-glucuronidase in the tumor microenvironment this has also been linked to poorer prognostic outcomes (13,14). This approach utilizing β-glucuronidase has previously been used and tested in vivo for targeted cancer therapy (15,16). Therefore, the presence of β-glucuronidase outside of the cellular environment could serve as a biomarker for the early detection of lung cancer and is a promising target for an EVOC probe approach.
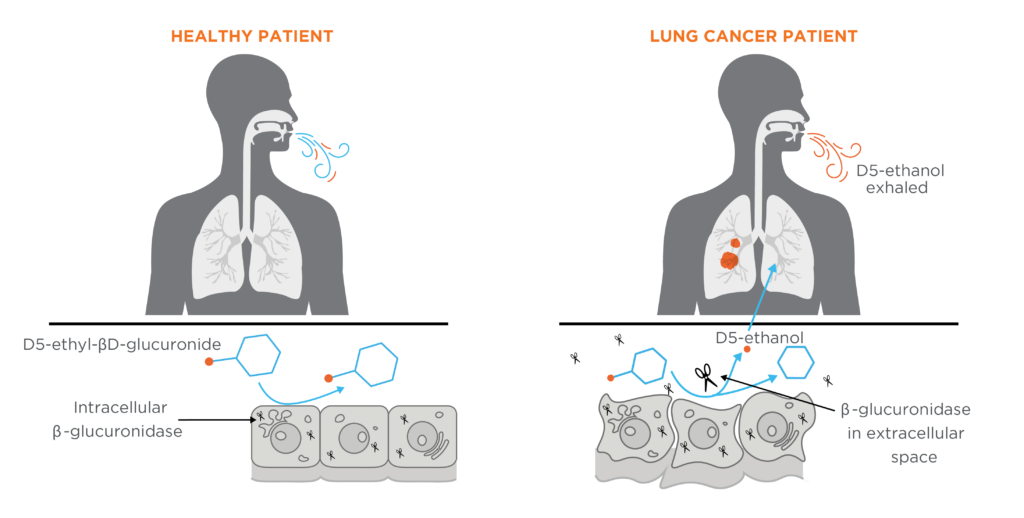
To exploit this mechanism, the probe D5-ethyl-βD-glucuronide was created. This probe can be metabolized by the β-glucuronidase enzyme to produce D5-ethanol. In someone without lung cancer, the D5-ethyl-βD-glucuronide probe will come into contact with the cells in their lungs. There is not normally β-glucuronidase enzyme present outside of the cells (extracellular), and therefore the probe should not be metabolized into D5-ethanol. If there is cancer in the lungs, the D5-ethyl-βD-glucuronide probe will come into contact with extracellular β-glucuronidase around the tumor and be metabolized into D5-ethanol which can subsequently be exhaled in the breath. Therefore, presence of D5-ethanol in the breath in response to administration of the probe could provide a reliable indicator for lung cancer, and potentially boost the signal to detect even small tumors. Potential also exists for such a breath test to be used to differentiate benign from malignant lung nodules identified through incidental findings or LDCT screening.
EVOLUTION Trial progress
Results from Phase 1
Results from Phase 1 of the EVOLUTION trial demonstrated that the molecular pathway targeted by the D5-ethyl-βD-glucuronide probe could indeed produce D5-ethanol, and provided crucial safety data, and evidence to support further test development. The EVOLUTION trial is currently at Phase 2, and is assessing the diagnostic performance of the test – enabling optimization of the probe through refinement of the breath testing protocol. Supporting this, the study has been designed to allow differentiation between individuals with lung cancer and relevant contrast groups (e.g. COPD) representative of the clinical populations in which the test is intended to be used.
Catch up on our recent news and data involving the EVOLUTION trial:
BBC NEWS: Royal Papworth Hospital: First-of-its-kind lung cancer test trialled
References
1. SEER [Internet]. [cited 2023 Sep 11]. Cancer Statistics Review, 1975-2015 – Previous Version – SEER Cancer Statistics Review. Available from: https://seer.cancer.gov/archive/csr/1975_2015/index.html
2. Association AL. State of Lung Cancer | Key Findings [Internet]. [cited 2024 Mar 28]. Available from: https://www.lung.org/research/state-of-lung-cancer/key-findings
3. Survival for lung cancer [Internet]. [cited 2024 Mar 28]. Available from: https://www.cancerresearchuk.org/about-cancer/lung-cancer/survival
4. Association AL. Lung Cancer Trends Brief [Internet]. [cited 2024 Mar 28]. Available from: https://www.lung.org/research/trends-in-lung-disease/lung-cancer-trends-brief
5. Arnold H. The Lung Cancer Policy Network. 2022 [cited 2024 Mar 28]. Out now — Lung cancer screening: learning from implementation. Available from: https://www.lungcancerpolicynetwork.com/the-lung-cancer-policy-networks-inaugural-report/
6. Gaude E, Nakhleh MK, Patassini S, Boschmans J, Allsworth M, Boyle B, et al. Targeted breath analysis: exogenous volatile organic compounds (EVOC) as metabolic pathway-specific probes. J Breath Res. 2019 May;13(3):032001. DOI: 10.1088/1752-7163/ab1789
7. Ferrandino G, Ricciardi F, Murgia A, Banda I, Manhota M, Ahmed Y, et al. Exogenous Volatile Organic Compound (EVOC®) Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis and Reveals Signs of Portal Hypertension. Biomedicines. 2023 Nov;11(11):2957. DOI: 10.3390/biomedicines11112957
8. Owlstone Ltd. Safety and Dose Ranging Study for OWL-EVO1 as a Lung Cancer EVOC® Probe [Internet]. clinicaltrials.gov; 2023 Dec [cited 2024 Jan 1]. Report No.: NCT05510674. Available from: https://clinicaltrials.gov/study/NCT05510674
9. Owlstone Ltd. Diagnostic Accuracy Study for OWL-EVO1 as a Lung Cancer EVOC® Probe [Internet]. clinicaltrials.gov; 2024 Mar [cited 2024 Jan 1]. Report No.: NCT06193239. Available from: https://clinicaltrials.gov/study/NCT06193239
10. Bosslet K, Straub R, Blumrich M, Czech J, Gerken M, Sperker B, et al. Elucidation of the mechanism enabling tumor selective prodrug monotherapy. Cancer Res. 1998 Mar 15;58(6):1195–201. LINK: https://pubmed.ncbi.nlm.nih.gov/9515805/
11. Juan TY, Roffler SR, Hou HS, Huang SM, Chen KC, Leu YL, et al. Antiangiogenesis targeting tumor microenvironment synergizes glucuronide prodrug antitumor activity. Clin Cancer Res. 2009 Jul 15;15(14):4600–11. DOI: 10.1158/1078-0432.CCR-09-0090
12. Mürdter TE, Sperker B, Kivistö KT, McClellan M, Fritz P, Friedel G, et al. Enhanced uptake of doxorubicin into bronchial carcinoma: beta-glucuronidase mediates release of doxorubicin from a glucuronide prodrug (HMR 1826) at the tumor site. Cancer Res. 1997 Jun 15;57(12):2440–5. LINK: https://pubmed.ncbi.nlm.nih.gov/9192823/
13. Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002 Mar;196(3):254–65. DOI: 10.1002/path.1027
14. Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998 Jan;152(1):83–92. LINK: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1858104/
15. Châtre R, Lange J, Péraudeau E, Poinot P, Lerondel S, Le Pape A, et al. In vivo synthesis of triple-loaded albumin conjugate for efficient targeted cancer chemotherapy. J Control Release. 2020 Nov 10;327:19–25. DOI: 10.1016/j.jconrel.2020.08.008
16. Renoux B, Fangous L, Hötten C, Péraudeau E, Eddhif B, Poinot P, et al. A β-glucuronidase-responsive albumin-binding prodrug programmed for the double release of monomethyl auristatin E. Medchemcomm. 2018 Dec 1;9(12):2068–71. DOI: 10.1039/c8md00466h