
Breath Biopsy Tests
Developing Breath Biopsy for clinical applications in early detection and precision medicine
INTRODUCTION
A Robust Pipeline for Rapid Biomarker Development and Test Delivery
Through the development of informative breath biomarkers into Breath Biopsy® tests for use in the clinic and clinical research, we aim to realize our mission of saving 100,000 lives and $1.5B in healthcare costs by applying our expertise in breath analysis to the areas of greatest clinical need. We undertake our own self-directed research to develop clinical breath tests in areas such as gastrointestinal disease and lung cancer, and we also provide support to researchers to incorporate breath analysis throughout the research journey.
Once the breath biomarkers of interest have been identified and validated, a breath test to be utilized in the clinic can be developed, which Owlstone Medical can support. We have our own hydrogen and methane breath tests for diagnosing and monitoring gastrointestinal conditions like small intestinal bacterial overgrowth (SIBO) and carbohydrate malabsorption conditions for the NHS in the UK through our patient-centric brand OMED Health. We are developing at-home breath devices, and so we have the expertise to potentially develop fit-for-purpose breath tests for volatile biomarkers that are of interest. It is also possible that these breath tests can be developed and deployed as at-home devices to support de-centralized trial designs with portable real-time breath collection and analysis.
In addition to the development of technologies to allow breath analysis, we have also focused on the development of breath tests for clinical and diagnostic use in the following disease areas:
Digestive Health
Breath Tests for Digestive Health Conditions
Over one billion people worldwide are affected by gastrointestinal illness, and symptoms can range from mild and infrequent, to severe and chronic with significant impacts on quality of life. Despite the range of illnesses that can affect gastrointestinal health, symptoms are largely similar, so achieving a diagnosis can take several years. The gut microbiome is thought to have an important impact on the development of disease, particularly of the gastrointestinal tract, and are responsible for producing a significant number of volatile compounds found in breath such as the short-chain fatty acids (SCFAs). The gut microbiome also produces informative gases like hydrogen, and methane, that can be measured in exhaled breath, and used for diagnostic purposes.
Through our patient-centric brand OMED Health, we are providing breath tests that aid in the diagnosis of SIBO, lactose intolerance, and fructose intolerance to the NHS. We have also developed an at-home hydrogen and methane breath device to support real-time longitudinal monitoring. These devices are suitable for diagnostic and research purposes, and can support decentralized trial designs as well as enable better patient monitoring for better, more personalized treatment plans.

Our vision is to develop a menu of Breath Biopsy Digestive Health tests and other tools to monitor breath composition. These could aid in research (particularly involving the microbiome), assist clinicians in making diagnoses, and for patients to better manage their day-to-day symptoms, and understand the impact of diet and other lifestyle factors on their digestive health.
Liver Disease diagnosis
Breath Tests for the Diagnosis of Liver Disease
Chronic liver diseases, such as metabolic dysfunction-associated steatotic liver disease (MASLD), that are driven by poor diet and low exercise have quietly reached epidemic levels across both adults and children. With timely, early intervention the liver can heal itself, but unfortunately, most patients are only diagnosed at the stage of cirrhosis when the liver is not able to perform physiological functions and the damage has become permanent. The liver is highly metabolically active making it a prime source of volatile metabolites found in breath.
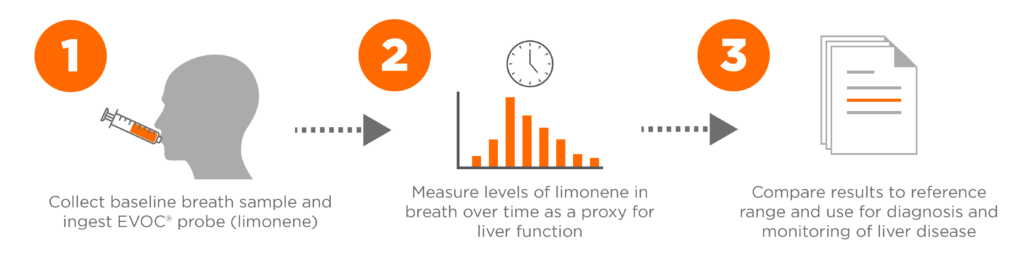
We have therefore developed a liver disease breath test, LIBRA®, to enable earlier detection when lifestyle changes and therapeutics can be effectively deployed. LIBRA® uses limonene as an exogenous VOC probe (EVOC® Probes), to provide a way to measure the activity of enzymes linked to liver function. LIBRA has already demonstrated success in the detection of cirrhosis with limonene bioavailability correlated to MELD score and fibrosis indicators, as well as signs of portal hypertension – demonstrating the clinical usability .
LUNG CANCER SCREENING
Breath Tests for the Early Detection of Lung Cancer
Globally, lung cancer is the most common cancer in the world with over 2 million diagnoses estimated in 2020. If detected early, the chance of surviving cancer is ten times higher, and the cost of treatment is ten times lower than in the later stages. Therefore, developing new ways to detect cancer early is the best way to improve cancer survival.
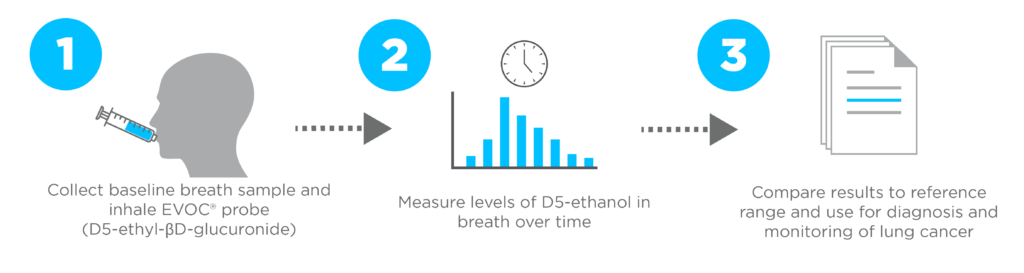
Through the EVOLUTION trial, our Breath Biopsy Lung Cancer test is currently in stage 2 of clinical trials, and involves an exogenous volatile organic compound (EVOC®) probe approach for the detection of lung cancer. The current probe being trialed is D5-ethyl-βD-glucuronide (D5-EthGlu), which is metabolized by β-glucuronidase, an enzyme found in the tumor microenvironment around lung cancer cells. This cleaves the probe releasing D5-ethanol, therefore serving as a unique biomarker of lung cancer via exhaled breath analysis.
Breath Review: Breath VOCs as a biomarker platform in disease areas
Read our paper highlighting the technologies developed to overcome challenges in breath analysis and updates on the use of breath analysis in various disease areas
