The challenge: Finding a Needle in a Haystack
Breath is highly complex, containing hundreds, even potentially thousands of volatile organic compounds (VOCs). The VOCs detected in breath span a wide breadth of chemical classes, including aromatic hydrocarbons, alcohols, ethers, esters, aldehydes, alkanes, alkenes, and ketones. Exhaled breath also has a unique property in that it is comprised of air that has been inhaled immediately prior from the environment, as well as a small portion of air that was already in the lungs (residual volume). The VOCs detectable in the breath can therefore originate from the background air that was inhaled immediately prior, or from internal physiological processes that have volatilized from the blood at the alveolar membrane, or directly from the respiratory tissues. The VOCs from internal processes are mostly present at very small concentrations, and therefore picking up meaningful signatures that arise from key metabolic processes that relate to disease is a bit like finding a needle in a haystack, amongst those that have arisen from the background air.
Adding another layer to this complexity is that VOCs genuinely arising from the underlying physiology can be split into two categories: endogenous VOCs that arise from human metabolic processes (such as inflammation), or exogenous VOCs that arise from external sources (such as from the gut microbiome, or from the diet) (Figure 1).
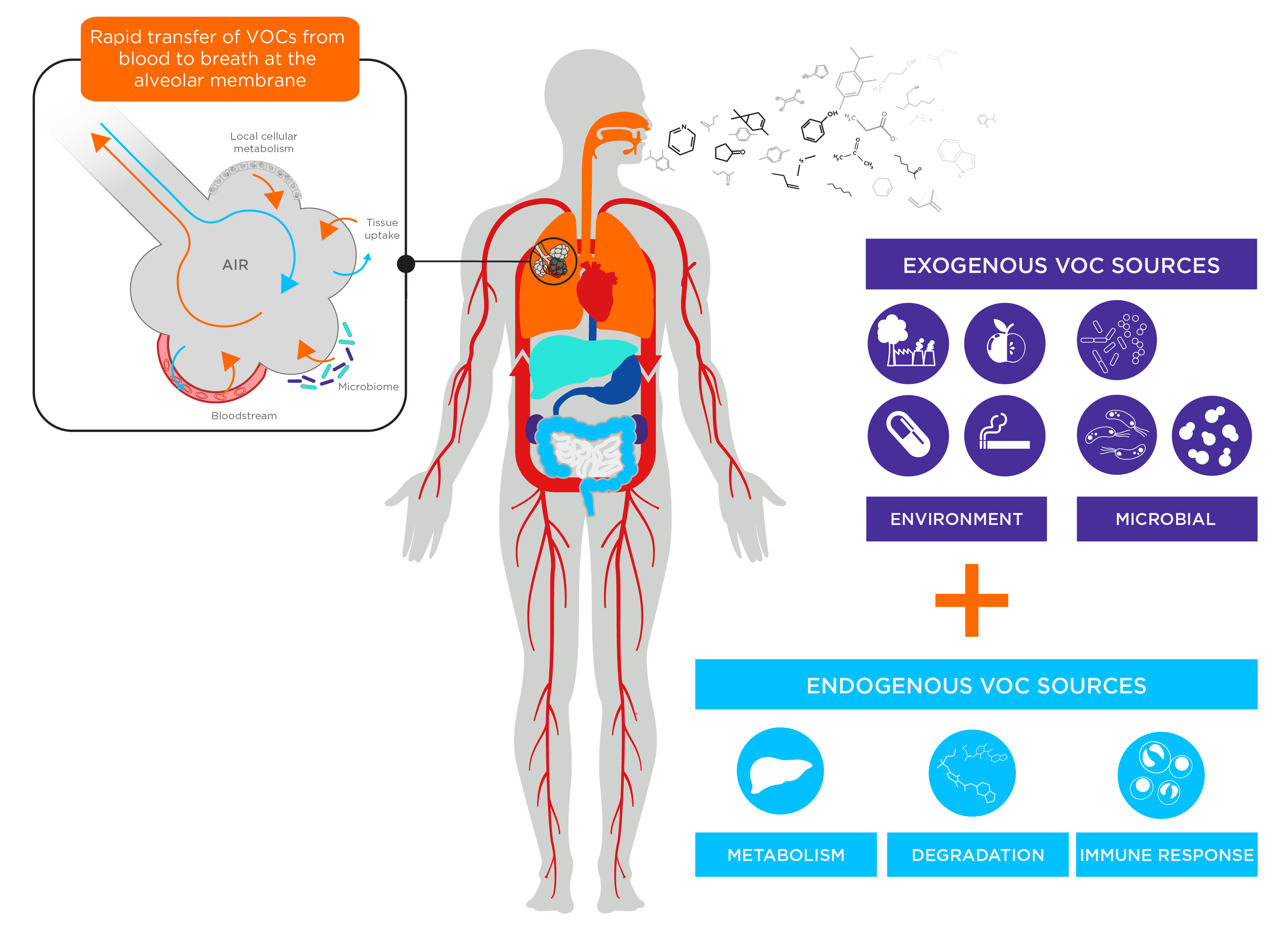
It must be noted that ‘background’ VOCs that have been inhaled immediately before sampling and therefore unrelated to underlying physiology can be distinct from exogenous VOCs, which are VOCs that have originally been introduced into the body via external sources such as diet.
Exogenous VOCs in the breath can be very informative, as the way the body interacts with these VOCs can inform on the function of internal metabolic processes, and VOC products from microbial metabolism from the microbiome can provide key insights into health and disease. This sparked an idea. If we want to highlight a specific pathway for diagnostic or clinical purposes, could we introduce an exogenous compound that interacts with a specific pathway, one that we know results in the production of a VOC detectable in breath, and use this to our advantage?
From this hypothesis came the exogenous volatile organic compound (EVOC®) probe approach.
Example research applications of EVOC Probes
We are currently focusing on the development of EVOC Probe-based Breath Biopsy applications to support research taking place in academic, clinical, and pharmaceutical settings. Three areas of focus are research in:
1. Liver Disease
Once ingested, most exogenous VOCs introduced into the body undergo metabolism in the liver and excretion via urine, but depending on how fast they are metabolized, a small fraction of the ingested dose remains unchanged and can circulate in the bloodstream and be excreted via the breath. For those with liver diseases, the associated metabolic processes do not function properly, potentially resulting in characteristic VOC breath composition changes. An EVOC probe given at a high dose could therefore be utilized to assess how the liver metabolizes the compound, and this could provide a direct readout of liver function that can be used for diagnostic purposes.
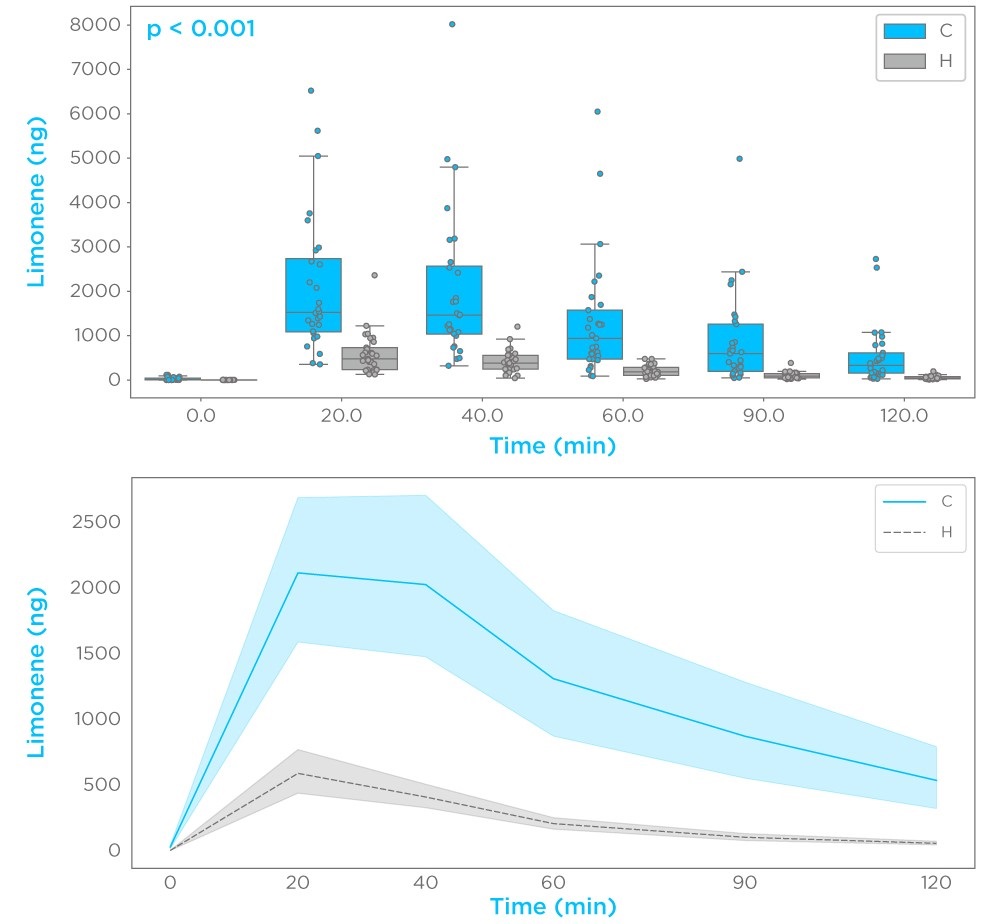
Limonene is an EVOC that is ingested mainly through the diet, particularly in citrus fruits and is accepted as a generally recognized as safe (GRAS) compound used in the pharmaceutical and food industry. Once ingested it is rapidly absorbed in the gastrointestinal (GI) tract, where it is distributed to the body, especially in the richly perfused organs, and mainly accumulates in the liver, with a small fraction excreted unchanged in the breath after acute exposure. We have previously shown that the use of limonene as an EVOC probe combined with dynamic breath testing over time can significantly enhance the diagnostic performance of a breath test for cirrhosis compared to static testing, and that the characteristic changes of limonene abundance in the breath for those with cirrhosis compared to controls is correlated with disease severity (Figure 3). This opens up the possibility of using limonene as a dynamic EVOC breath test to monitor the success of therapeutic interventions.
2. Lung Cancer
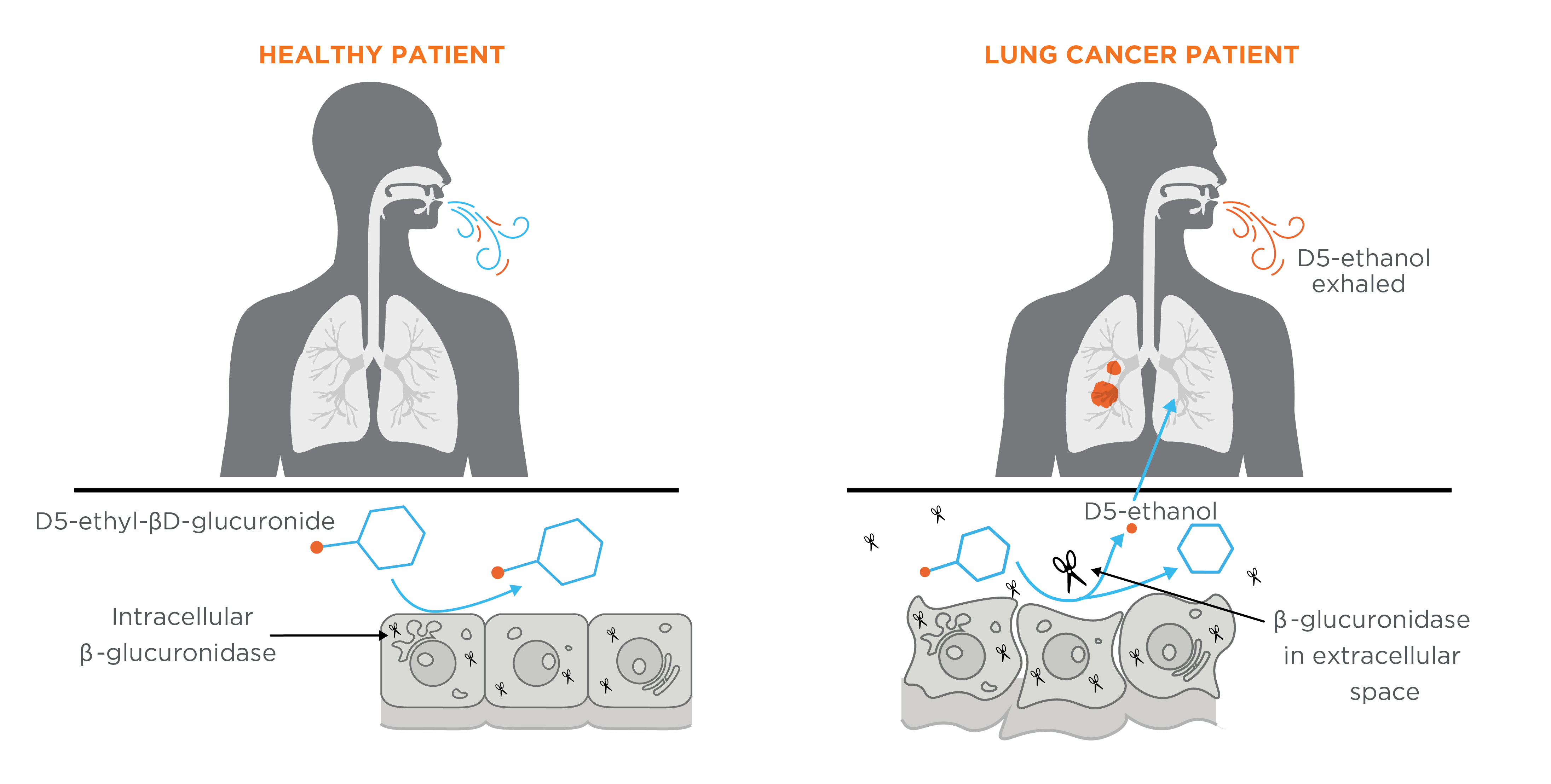
An EVOC prove approach to lung cancer is currently being tested as part of our EVOLUTION clinical trial. The current EVOC approach involves targeting cancer-associated β-glucuronidase enzymes. Under normal conditions, the enzyme β-glucuronidase is present within cells in the lysosome and is responsible for the catalytic hydrolysis of β-d-glucuronides. Research in humans and mice has pointed towards β-glucuronidase being differentially expressed in the tumor microenvironment (includes tumor cells and the extracellular space with surrounding cells, blood vessels, and tissues) of many types of lung cancer, and interestingly, high levels of β-glucuronidase in the tumor microenvironment this has also been linked to poorer outcomes.
This approach utilizing β-glucuronidase has previously been used and tested in vivo for targeted cancer therapy, and so is a prime candidate pathway to target with a designed EVOC probe to support the development of a breath test for lung cancer. To exploit this mechanism, the probe D5-ethyl-βD-glucuronide was created.
This probe can be metabolized by the β-glucuronidase enzyme to produce D5-ethanol, a volatile reporter compound In someone without lung cancer, the D5-ethyl-βD-glucuronide probe will come into contact with the cells in their lungs. Normally there is no β-glucuronidase enzyme present outside of the cells (extracellular), and therefore the probe should not be metabolized into D5-ethanol. If there is cancer in the lungs, the D5-ethyl-βD-glucuronide probe will come into contact with extracellular β-glucuronidase around the tumor and be metabolized into D5-ethanol which can subsequently be exhaled in the breath (Figure 4). Therefore, the presence of D5-ethanol in the breath in response to administration of the probe could provide a reliable indicator for lung cancer, and potentially boost the signal to detect even small tumors, where the endogenously originating signal would usually be negligible amongst other VOCs sampled from the breath.
3. Drug Metabolism
Approximately 40% of drugs do not work as expected when prescribed, leading to adverse events or to the drug not providing the therapeutic benefit expected. This is largely due to drug metabolism, the processing of drugs into their active forms, and the breaking down of drugs for excretion. When this process goes wrong, a drug can build up in the body to toxic levels or the active form never reaches sufficient levels to have the desired therapeutic effect (Figure 5).
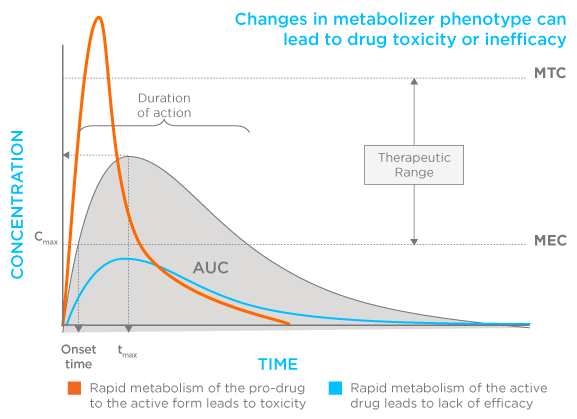
The vast majority of small molecule drugs are metabolized by a class of enzymes called the cytochrome P450s, of which four are responsible for approximately 70% of activity, including metabolizing some of the most highly prescribed medications including Lipitor (cardiovascular), Zoloft (mental health), and Tramadol (pain).
Knowledge of a patient’s drug metabolism status allows clinicians to make more informed decisions as to which drugs to prescribe and at what dose. As a result, the pharmacogenomics market, testing a person’s genes to look for polymorphisms that predict how that individual would metabolize a drug, is growing rapidly. The problem with this approach is that genes are only one measure of risk, but not a complete measure of how a patient would actually metabolize a drug. For example, genotyping cannot account for diet, smoking, or other drugs a patient may be taking. A mechanism to get a quantitative readout on key drug metabolic pathways in individual patients is through a targeted EVOC probe to specific pathways of interest.
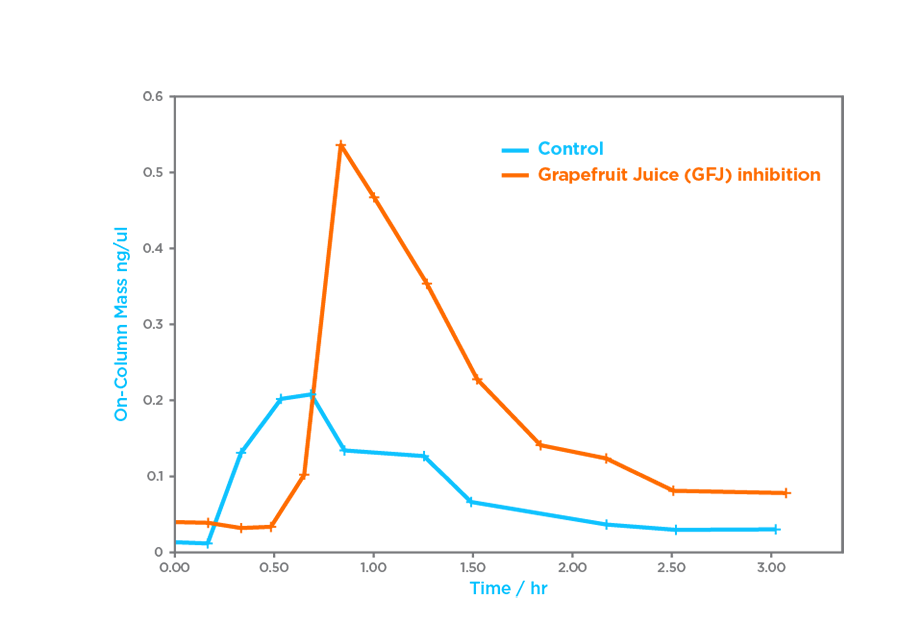
For example, EVOC Probes that are metabolized by the same P450 enzymes as common medications could be used to anticipate the rate of drug processing and breakdown and produce a phenotypic profile that, if sufficiently accurate, could be used to modulate drug dosage for safer and more effective results. In the example below, grapefruit juice, which is well known to inhibit P450 enzymes (which is why many medications come with a warning not to eat grapefruit, or drink grapefruit juice while taking them) is used to assess the impact on specific breath VOCs (such as eucalyptol) after inhibition of P450 enzymes that would usually metabolize them (Figure 6).