Exploring the Link Between the Microbiome and the Immune System
Published on: 8 Feb 2024
The gut microbiome is a community of microbial organisms colonizing the gastrointestinal tract and typically exists in a symbiotic relationship with our body. Our microbiome has co-evolved with us and has developed a multifaceted relationship with many biological processes occurring within our body, including a particularly close bidirectional interaction with our immune system.
Many of the metabolites that are produced by the gut microbiome are volatile organic compounds (VOCs) which can be measured in exhaled breath and can therefore be quantified using non-invasive breath analysis. This can enable a greater understanding of the processes involved in the relationship between our microbiome and immune system1.
Gut Microbiome and Immune System Interactions – Impacts on Health and Disease
As the GI tract is constantly exposed to the environment as we eat and drink, our “good” bacteria, that comprise our microbiome, are in frequent competition with potentially disease-causing pathogens as they both vie for space and nutrients in our gut. As such, our microbiomes have evolved to fend off pathogens and “educate” the immune system so that we maintain a healthy balanced environment.
These interactions have created an interdependent relationship between our immune system and microbiome, controlled by a variety of immune receptors and signalling pathways.
A myriad of interactions achieve this, including reinforcing components of the epithelial barrier, stimulating the production of antibodies and lymphocytes, and modulating the inflammatory response. Further complicating this relationship is the effect of dietary factors which may upregulate or downregulate these interactions1.
Dr. Andrew Kau from Washington University School of Medicine recently presented at the Breath Biopsy Conference 2024, on ‘The Breath Volatilome is Shaped by the Gut Microbiota’. You can watch Dr. Kau’s talk, as well as the rest of the presentations from the conference here.
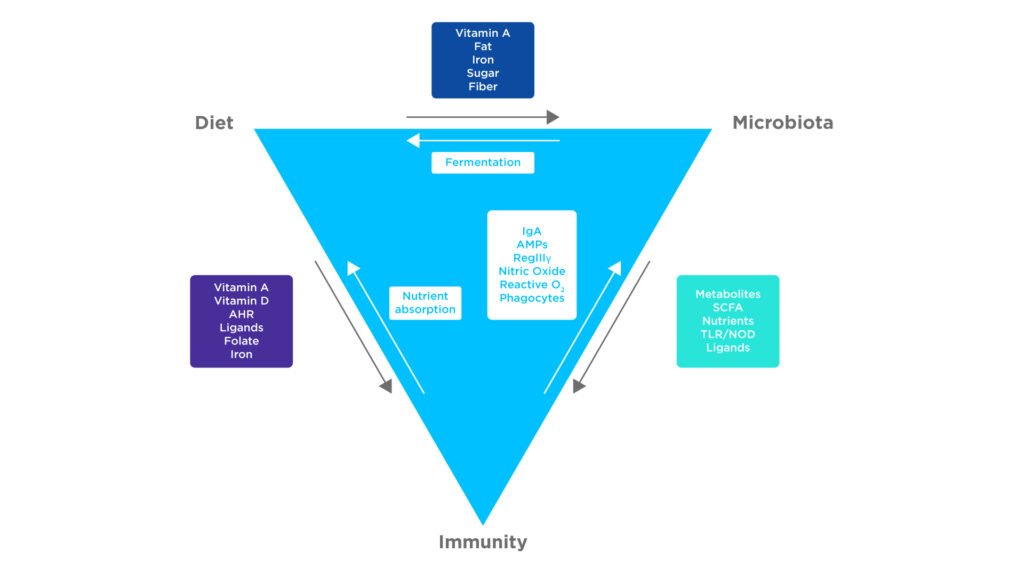
Figure 1: There are many factors affecting the interactions between the microbiome, immune system and diet. Adapted from Belkaid Y et al1.
Some key metabolites that regulate immune cells and immune tolerances are short chain fatty acids (SCFAs). SCFAs are produced through the fermentation of dietary fibers by the microbiome, absorbed by the intestines, before being transported throughout the circulatory system. Many of these SCFAs are volatile and therefore can be measured in breath.
SCFAs, particularly propionate and butyrate, act as histone deacetylase inhibitors, the inhibition of which increases the activity of T and B cells and improves tissue barrier function. This occurs through the upregulation of gene expression and cell activation pathways as a consequence of increased protein acetylation. Specifically, increasing IL-10 expression in T cells and macrophages and IL-22 and IL-17 during an immune response.
In addition, the increased production of retinoic acid, through the action of SCFAs on retinaldehyde dehydrogenase, upregulates the production of regulatory T cells. The results of these factors are a reduction in inflammation, the maintenance of the gut barrier, and the suppression of autoimmune diseases2.
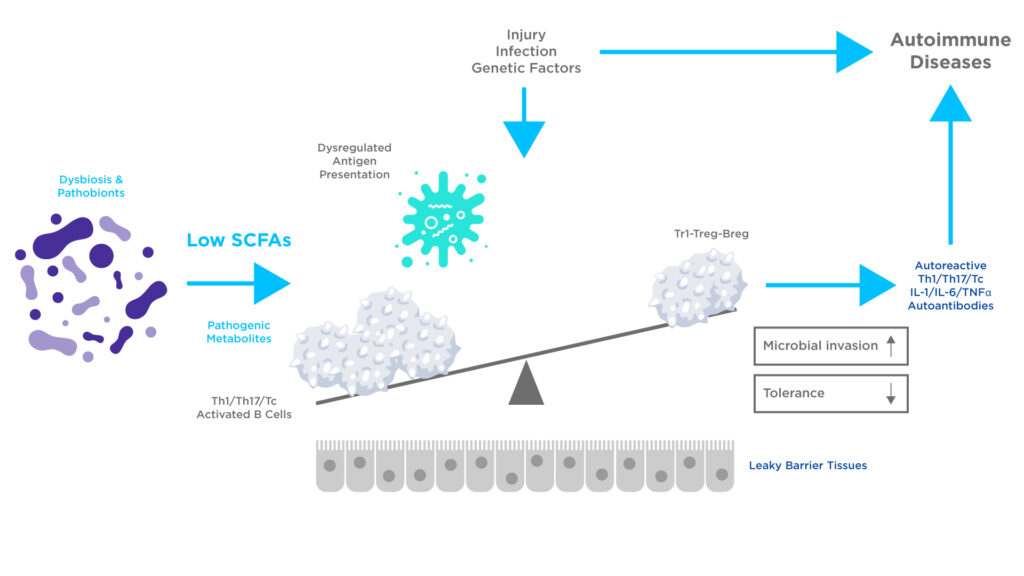
Dysbiosis of the microbiome can reduce SCFAs production, which may alter immune function, including the generation of autoreactive effector lymphocytes. An altered microbial composition may also produce harmful and inflammatory metabolites that can increase the permeability of the intestinal barrier. Such microbiome alterations have been linked to inflammatory bowel disease (IBD) and autoimmune disease1, 2.
Figure 2: The effects of dysbiosis may lead to altered production of microbial metabolites, leading to reduced SCFA levels. This deficiency in SCFA production has the potential to alter the functions of Antigen-Presenting Cells (APCs), T cells, and B cells. Harmful metabolites can inflict damage on tissue and enhance the inflammatory activities of immune cells leading to autoimmune disease. Adapted from Kim CH et al2.
For example, gut dysbiosis has been recorded in type 1 diabetes patients. The inhibition of histone deacetylases by SCFAs typically protects the pancreatic islet cells from the damage caused by inflammatory metabolites. However, the compromised immune tolerance in patients with insufficient levels of SCFAs makes them more susceptible to an autoimmune response. In fact, SCFA profile and microbiome diversity are reported as abnormal in various autoimmune diseases including rheumatoid arthritis, lupus, and multiple sclerosis, amongst others reflecting the microbiome’s influence extending beyond the gut2.
Implications for Treatment and Research
A greater understanding of the interactions between the microbiome and the immune system could provide a valuable basis for therapeutic and diagnostic development. As described in the examples above, the levels of SCFAs have significant effects on the pathogenesis of autoimmune diseases. Therefore, therapeutics targeting SCFA producing microbes could be a potential treatment for these conditions.
However, the complexity of the multifaceted regulatory pathways modulated by SCFAs and differences between individuals could lead to treatments being unsuccessful or even harmful. Therefore, further research on understanding the interplay between the microbiome, diet, and the immune system is required2.
Breath analysis provides a scalable, and non-invasive technique for metabolites including SCFAs to be measured. It can provide highly sensitive real-time quantification in response to various stimuli such as diet3 or therapeutic interventions and is well tolerated by patients, therefore suited for ongoing-monitoring and early detection.
At Owlstone Medical, our internal studies use our Breath Biopsy OMNI platform to monitor the metabolic response of the microbiome to various nutritional stimuli and identify those associated with diseases, including metabolic dysfunction-associated fatty liver disease (MAFLD). We aim to identify translatable biomarkers and develop clinical tests that can be used to develop therapeutic and diagnostic solutions.
To find out more about our work in this area you can view our research poster or read our blog article on the topic. If you are interested in incorporating breath analysis into your biomarker discovery workflow and would like to discuss it with our team, please get in touch.
References:
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014 Mar 27;157(1):121-41. doi: 10.1016/j.cell.2014.03.011.
- Kim CH. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell Mol Immunol. 2023 Apr;20(4):341-350. doi: 10.1038/s41423-023-00987-1.
- Neyrinck AM, Rodriguez J, Zhang Z, Nazare JA, Bindels LB, Cani PD, Maquet V, Laville M, Bischoff SC, Walter J, Delzenne NM. Breath volatile metabolome reveals the impact of dietary fibres on the gut microbiota: Proof of concept in healthy volunteers. EBioMedicine. 2022 Jun;80:104051. doi: 10.1016/j.ebiom.2022.104051
Microbiome Content Pack: exclusive content on how breath analysis can be used for gut microbiome research