
A non-invasive breath test to measure key aspects of liver function
The LIBRA® Liver Function Test is suitable for home-use: ideal for monitoring changes in liver function over time
INTRODUCTION
The liver plays a key role in human health, broadly responsible for metabolism, detoxification and protein synthesis. Dysfunction of the liver is associated with many conditions including obesity, diabetes, cardiovascular disease, kidney disease, and autoimmune conditions like lupus, as well as diseases of the liver (e.g. alcohol related liver disease).
Traditionally, liver function is measured with ‘liver function tests’ (LFTs) using blood samples taken in primary care settings. However, many of these are not true measures of how well the liver is performing its key functions instead they simply measure liver cell damage. Other tests report on the synthesis of key proteins (e.g. albumin), or natural products of liver degradation. However, these processes can be affected by multiple other factors, such as nutritional status and some kidney conditions, which can mask true changes in liver function. Additionally, some biomarkers take time to change in response to liver damage.
A key liver function is extracting compounds from the blood stream and metabolizing them to less harmful and more excretable forms. No regularly used liver function test currently measures this. Our new LIBRA® Liver Function Test allows for this to be measured with a convenient non-invasive breath test suitable for clinic and home use.
We know that liver function can improve in individuals with liver impairment, for example, with lifestyle changes and other interventions. Due to the non-invasive nature of the test, practical administration at home is possible. The LIBRA Liver Function test is therefore well-suited to analysing changes in liver function longitudinally to monitor for treatment success, or deterioration in liver metabolic function.
What is liver metabolic activity, and why is it important to look at?
The liver is the body’s major site of detoxification; it processes drugs, toxins and other small molecules into more excretable forms. The enzymes responsible are found in hepatocytes. In conditions where the liver is damaged (e.g. cirrhosis) we know there is reduced function of a variety of enzymes – due to both loss of hepatocytes and reduced enzyme transcription (1). For example, alcohol dehydrogenase is known to be reduced in liver disease (2). This reduced function contributes to the negative health outcomes of liver disease.
While loss of hepatocytes and reduced enzyme levels are features of late-stage liver disease, alterations in liver metabolic activity can also be seen in early-stage liver disease like MASH (3) and in other acute conditions – for example sepsis, liver inflammation, and ischemia (4).
What is extraction efficiency, and why is it important to look at?
For a compound to be metabolised in hepatocytes, it must first get there. Extraction efficiency relates to the ability of the liver to remove a compound from the blood to get it to the enzymes that then degrade it.
Changes in liver structure and blood flow are present in late-stage liver conditions like cirrhosis, which affects extraction efficiency, as well as earlier stage disease, including MASH and even obesity (5). Other conditions that affect blood flow to, and from the liver (e.g. heart failure) (6) can also impact extraction efficiency.
How is the LIBRA Liver Function Test Performed?
The LIBRA Liver Function Test is simple to use and can be performed in the home environment as well as in the clinic – in the morning is ideal – see Figure 1 for an overview.
After a short fast, the LIBRA oral solution is taken by the patient (this is sweetened for palatability) and contains ingredients widely found in food and confirmed to be safe (GRAS). After around 25 minutes, simple instructions for use direct participants to collect a breath sample. The LIBRA analytes are collected, concentrated and stabilised from the breath using specialised materials inside the collection device, enabling the collected breath samples to be sent using standard postal services at room temperature. They are analysed in the Owlstone Breath Analysis laboratory post-collection.
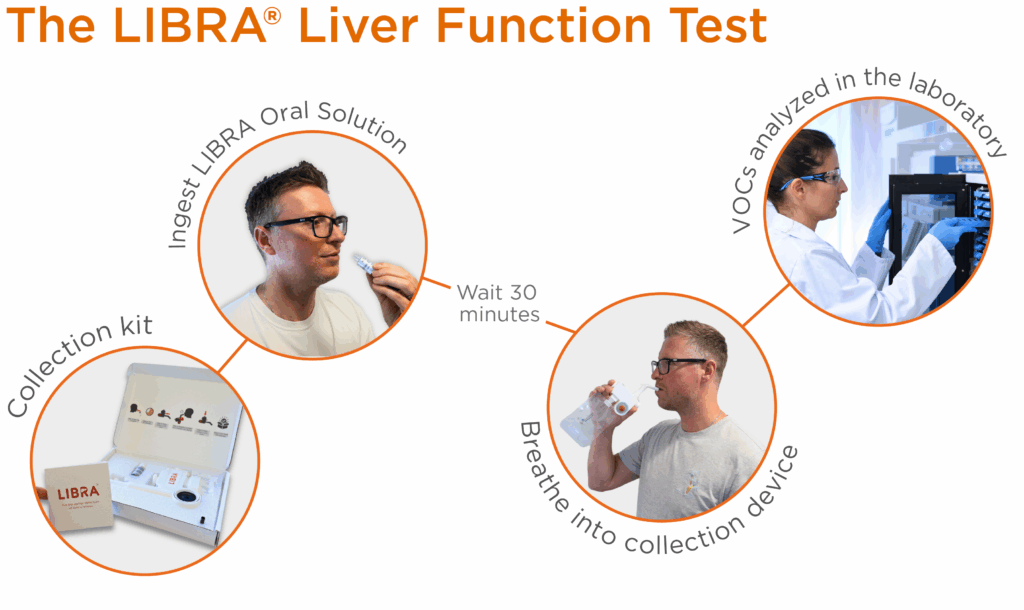
How does the LIBRA Liver Function Test Work?
The food safe compounds in the LIBRA oral solution are absorbed from the gut and processed in the liver. For some of these compounds, the rate of metabolism is dependent on the actual functional levels of certain liver enzymes – i.e related directly to liver metabolic activity. Other LIBRA compounds are more dependent on the access to the sites where they are metabolised – i.e. their levels in blood and rate of metabolism are related to extraction efficiency, which is influenced by liver structure and blood flow (shown in figure 2).
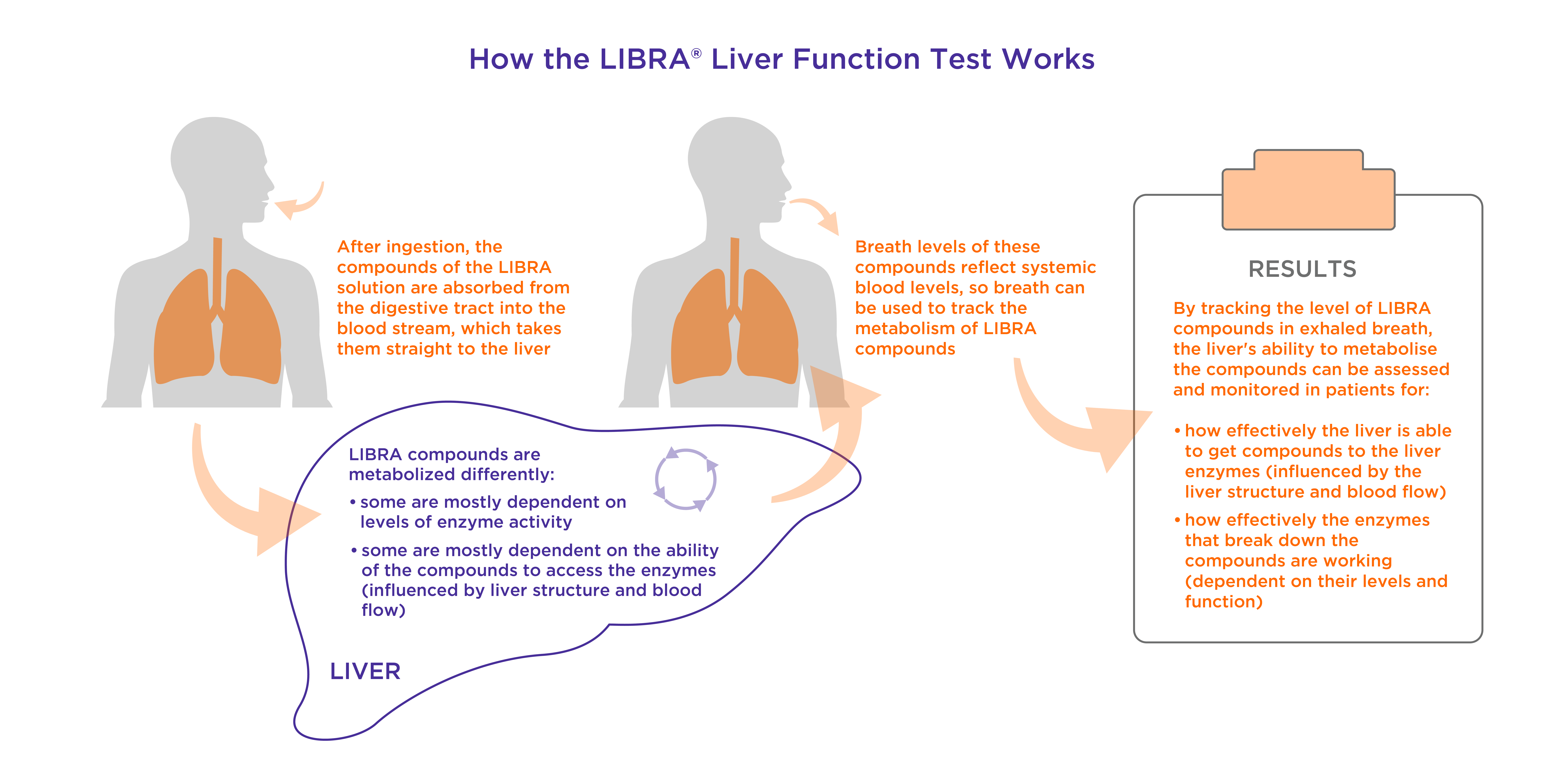
By analysing the levels of the LIBRA oral solution compounds and their metabolites in breath* at an appropriate point after ingestion of the LIBRA solution, we can get a measure of liver metabolism and extraction efficiency. This can be:
- compared between timepoints in the same person to understand changes in their personal liver function
- compared between different patient groups to compare levels of liver function in them
- compared to our reference ranges to get a read out of how well the liver is functioning versus e.g. healthy people or people with confirmed liver disease
* The levels of the LIBRA compounds found in breath are proportional to the concentration levels in blood – similar to the principles of an alcohol breathalyser
APPLICATIONS
Why use the LIBRA Liver Function Test for research?
The LIBRA Liver Function Test probes a liver function that is not investigated by other common liver tests. It relates to how an individual’s liver is actually doing one of its critical jobs. A true measure of liver function.

It gives an assessment at of the livers function at the time of the test (unlike other liver tests which report more on what the state was at an earlier point) – there is potential to report on more rapid changes in liver function.

Enables measurement of liver function in the home environment for easier and cheaper decentralised clinical trials.

Breath is non-invasive and easy to collect – it is ideal for doing longitudinal clinical studies and monitoring changes in liver function.
LIBRA CASE STUDy examples
Comparing Healthy and Cirrhotic Subjects
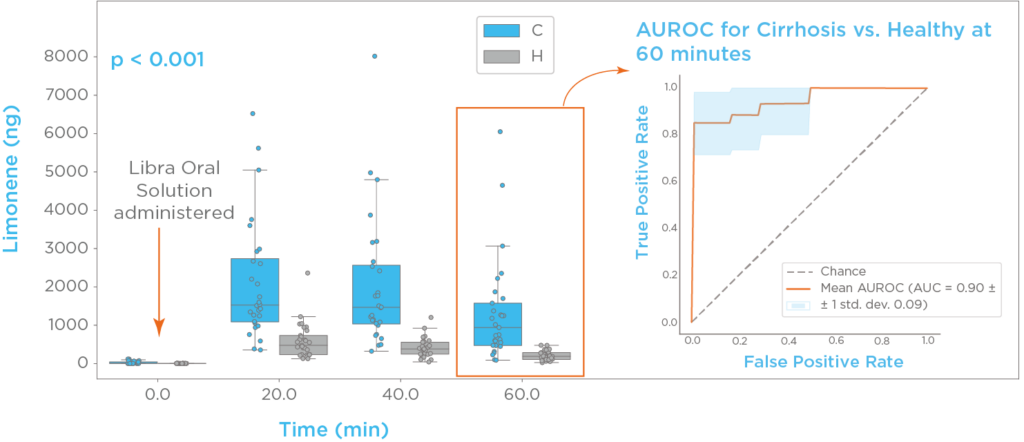
Limonene, a compound commonly found in citrus fruits, is present in the LIBRA oral solution. Thus, using the LIBRA Liver Function Test, limonene breath profiles can be generated for each participant. As seen in figure 3, limonene ingestion elicits a >100-fold spike of breath limonene compared to baseline. The investigated timepoints post-administration showed excellent classification performance. At 60 minutes we measured an area under the roc curve (AUROC) of 0.91, sensitivity of 0.83 ± 0.07, and specificity of 0.9 ± 0.06 (7).
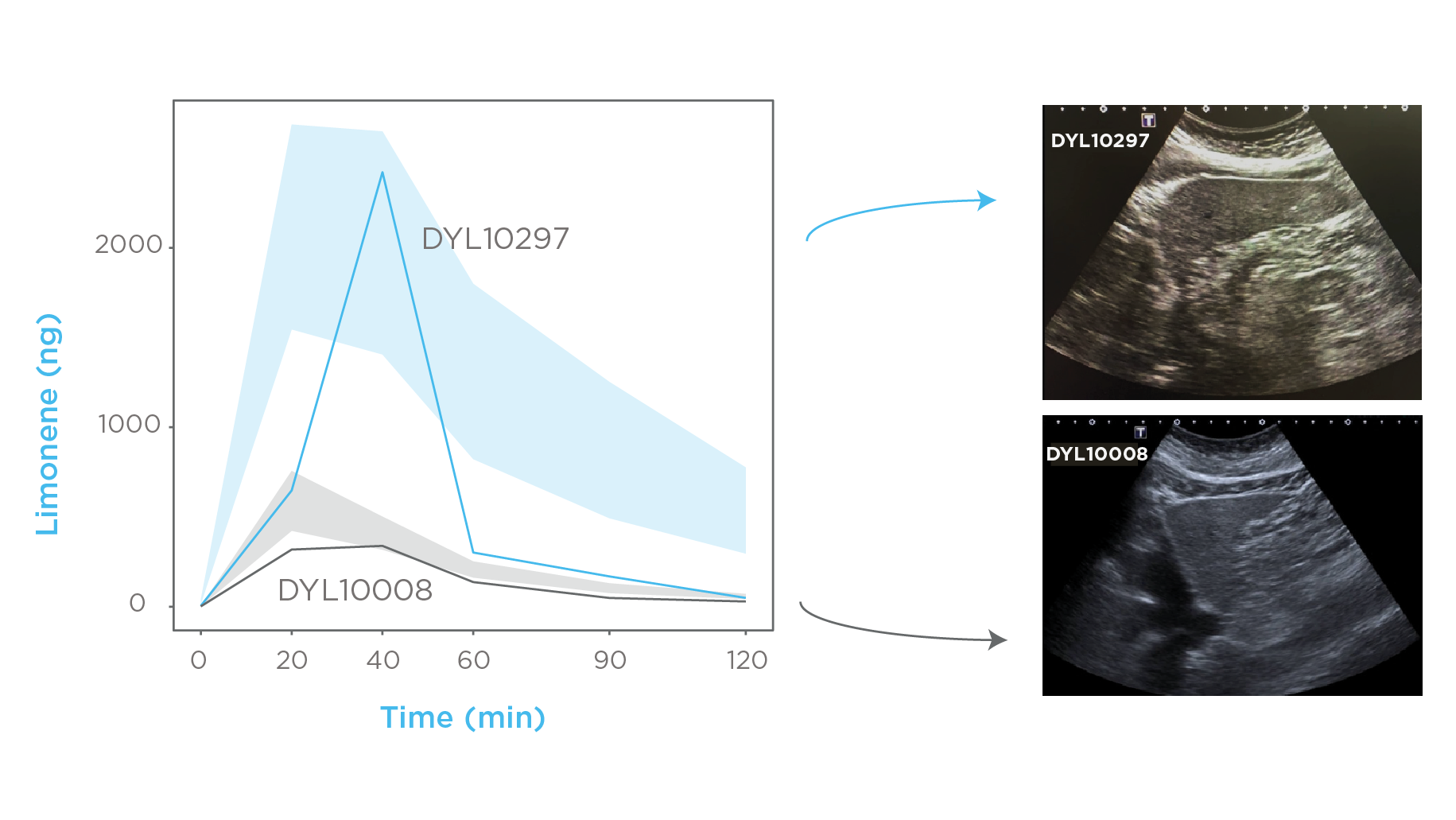
Ferrandino and colleagues effectively identified subjects who had been incorrectly allocated into the cirrhotic and healthy control groups. Figure 4 demonstrates that subject DYL10297 was initially recruited as a control but exhibited an abnormal breath profile. A follow-up ultrasound revealed previously unknown liver disease. Similarly, Subject DYL10008, classified in the cirrhosis group based on a three-year-old autoimmune hepatitis diagnosis, displayed a breath profile resembling that of healthy subjects. A further ultrasound confirmed cirrhosis regression, suggesting successful immunosuppressant treatment. These findings highlight LIBRA’s potential for both chronic liver disease detection and treatment monitoring.
Detecting Cirrhosis in a High Risk Population
In a recent unpublished study, LIBRA identified subjects with cirrhosis. The cohort included high-risk individuals who had symptoms of cirrhosis. 78 of 147 individuals were diagnosed with early compensated cirrhosis across Chile, the United Kingdom, and the United States. Figure 5 shows the classification performance, with an area under the curve (AUC) of 0.82, sensitivity of 0.73 and specificity of 0.73 (at Youden index).
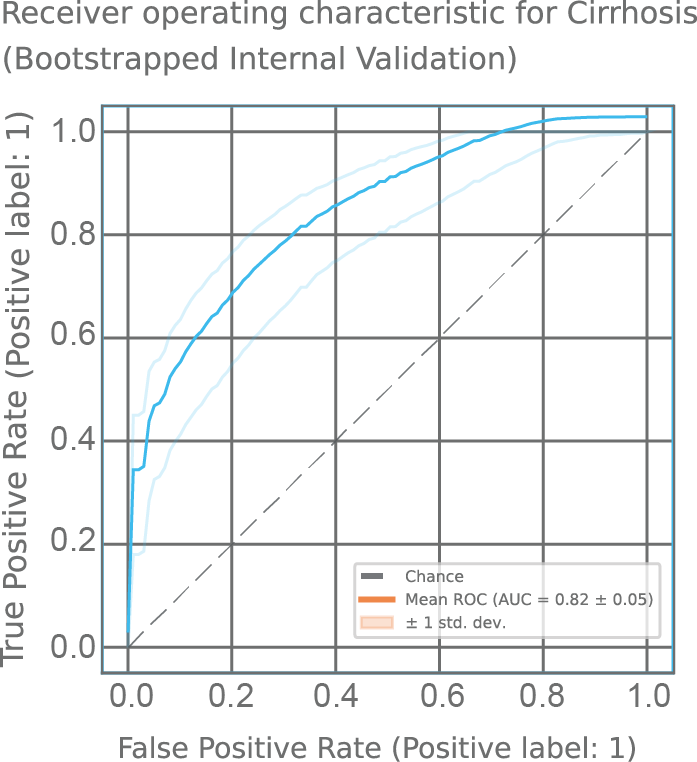
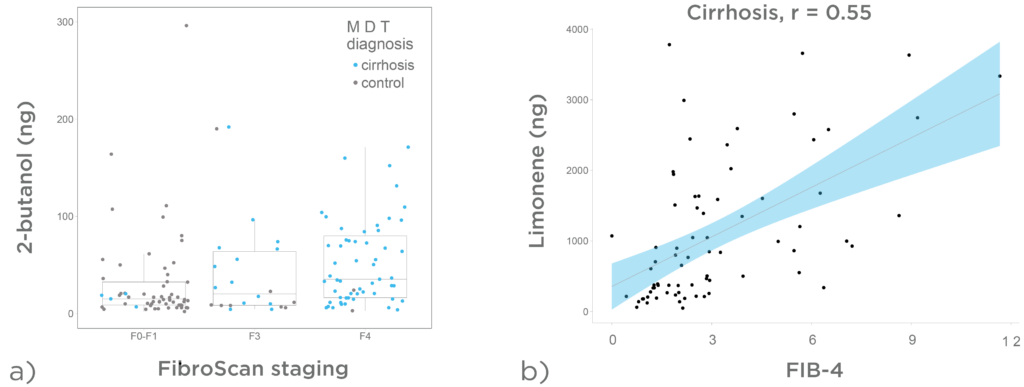
Figure 6a demonstrates that a progressive increase of LIBRA target analytes on breath was observed across the spectrum of liver fibrosis estimated using FibroScan®. In figure 6b, we can see breath limonene 15 minutes post administration of the LIBRA Oral Solution. There is a positive correlation between limonene levels and FIB-4 in subjects with cirrhosis.
Portal Hypertension
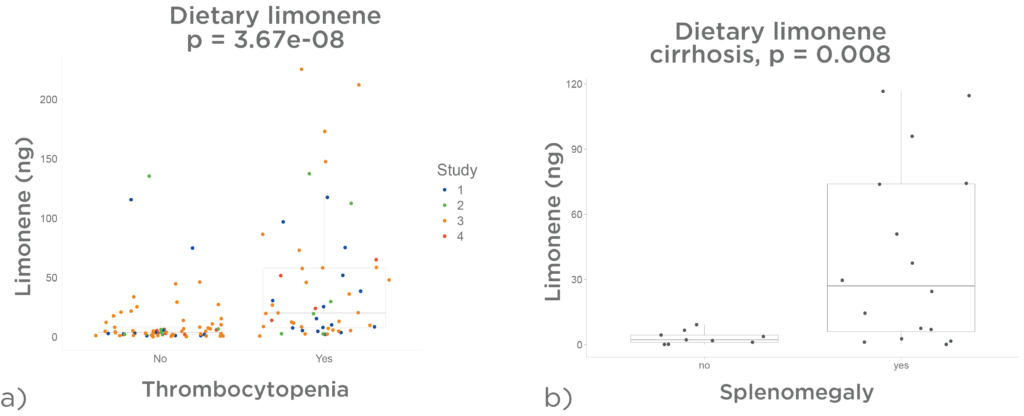
Dietary limonene is has been associated with signs of portal hypertension. Figure 7 a) is an analysis of 133 cirrhotic patients across four studies and revealed higher breath limonene levels derived from dietary exposure in individuals with thrombocytopenia (platelet count <150 × 10/L).
In an exploratory study of 29 cirrhotic patients, ultrasound evaluation of spleen size showed that those with splenomegaly (spleen >12 cm) exhibited increased breath limonene levels from random dietary exposure (figure 7 b).
Since thrombocytopenia and splenomegaly are established markers of portal hypertension, these findings suggest LIBRA’s potential for longitudinal monitoring, aiding in the early detection of portal hypertension and timely intervention.
Chronic Liver Disease
A progressive rise in limonene through the chronic liver disease spectrum has been seen in four independent studies conducted by Owlstone Medical. These looked at limonene levels found on breath from dietary intake (no intervention). Figure 8 illustrates that healthy individuals display low levels of breath limonene, whilst pre-cirrhotic and cirrhotic patients show increasingly high levels. Changes in VOCs are thus detectable at pre-cirrhotic stages, suggesting early biomarkers for liver dysfunction.
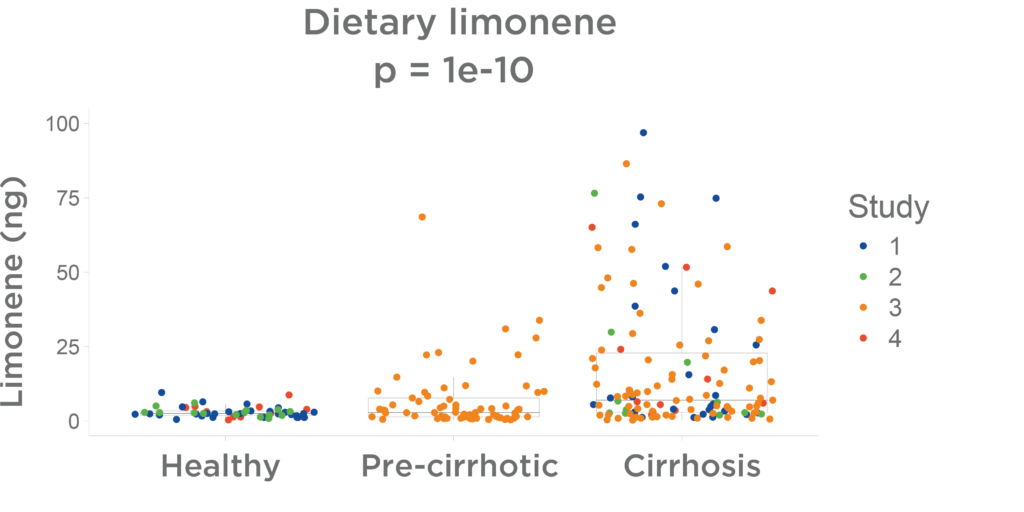
The LIBRA Liver Function Test may facilitate early identification of individuals with chronic liver disease before the cirrhosis stage, enabling early intervention. The progressive nature of VOC alterations could serve for monitoring liver function improvement.
Would you be interested in using the LIBRA Liver Function Test in your research? Do not hesitate to get in touch to learn more about how we can help.
Quick Start Guide: Everything you need to know about how breath analysis can be used in liver disease research
References
1. Christoph G Dietrich OG, Tze AG. Molecular changes in hepatic metabolism and transport in cirrhosis and their functional importance. World Journal of Gastroenterology. 2016 Jan 7;22(1):72–88. doi: 10.3748/wjg.v22.i1.72
2. Vidal F, Perez J, Morancho J, Pinto B, Richart C. Hepatic alcohol dehydrogenase activity in alcoholic subjects with and without liver disease. Gut. 1990 Jun 1;31(6):707–11. doi: 10.1136/gut.31.6.707
3. Merrell MD, and Cherrington NJ. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metabolism Reviews. 2011 Aug 1;43(3):317–34. doi: 10.3109/03602532.2011.577781
4. Guo Y, Hu ,Bingfang, Xie ,Yang, Billiar ,Timothy R., Sperry ,Jason L., Huang ,Min, et al. Regulation of drug-metabolizing enzymes by local and systemic liver injuries. Expert Opinion on Drug Metabolism & Toxicology. 2016 Mar 3;12(3):245–51. doi: 10.1517/17425255.2016.1139574
5. Di Ciaula A, Carbone F, Shanmugham H, Molina-Molina E, Bonfrate L, Ministrini S, et al. Adiponectin involved in portal flow hepatic extraction of 13C-methacetin in obesity and non-alcoholic fatty liver. Eur J Intern Med. 2021 Jul;89:56–64. doi: 10.1016/j.ejim.2021.03.036
6. Xanthopoulos A, Starling RC, Kitai T, Triposkiadis F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC: Heart Failure. 2019 Feb 1;7(2):87–97. doi: 10.1016/j.jchf.2018.10.007
7. Ferrandino G, Ricciardi F, Murgia A, Banda I, Manhota M, Ahmed Y, et al. Exogenous Volatile Organic Compound (EVOC®) Breath Testing Maximizes Classification Performance for Subjects with Cirrhosis and Reveals Signs of Portal Hypertension. Biomedicines. 2023 Nov 1;11(11):2957. doi: 10.3390/biomedicines11112957