How Hydrogen-Methane Breath Tests Can Help Us Characterize Gut Dysbiosis in Liver Disease
Published on: 10 Jul 2025
The gut-liver axis is a bidirectional communication pathway that involves the movement of substances between the gut and the liver. Due to this connection, disruptions in one can significantly affect the other. The community of microorganisms residing in the gut, known as the gut microbiome, play a crucial role in this interplay. Thus, measuring gut microbial metabolites can provide key insights into gut health in patients with liver conditions.
The Gut Microbiome
A healthy gut microbiome contributes to many bodily functions including micronutrient production, protection from invading pathogens, supporting gut barrier integrity and influencing metabolic regulation, immune and inflammatory processes (1).
Dysbiosis occurs when there is an imbalance of microorganisms in the gut, such as in small intestinal bacterial overgrowth (SIBO) and intestinal methanogen overgrowth (IMO). Excess bacteria ferment dietary fibres producing gases like hydrogen, whilst a specific group of microbes, known as methanogenic archaea, consume four molecules of hydrogen to produce one molecule of methane. This process is crucial because methane itself has distinct pathophysiological effects (2,3). Both gases are absorbed into the bloodstream, enabling them to travel to the lungs, where gas exchange occurs, allowing them to be measured in exhaled breath.
Since human cells do not produce hydrogen and methane, breath testing for these microbial metabolites could provide a crucial, non-invasive window into the complex interactions between the gut microbiome and the liver.
Diagnostic Accuracy of Breath Tests in Cirrhosis Patients
An association between gut dysbiosis and liver disease was first observed in a 1957 study that found significantly higher levels of bacteria in the small intestine of cirrhosis patients with none or very few detected in healthy people (4). A systematic review has since confirmed this association, yet different ways of testing for gut microbiome overgrowth have resulted varied outcomes, as will be discussed (5).
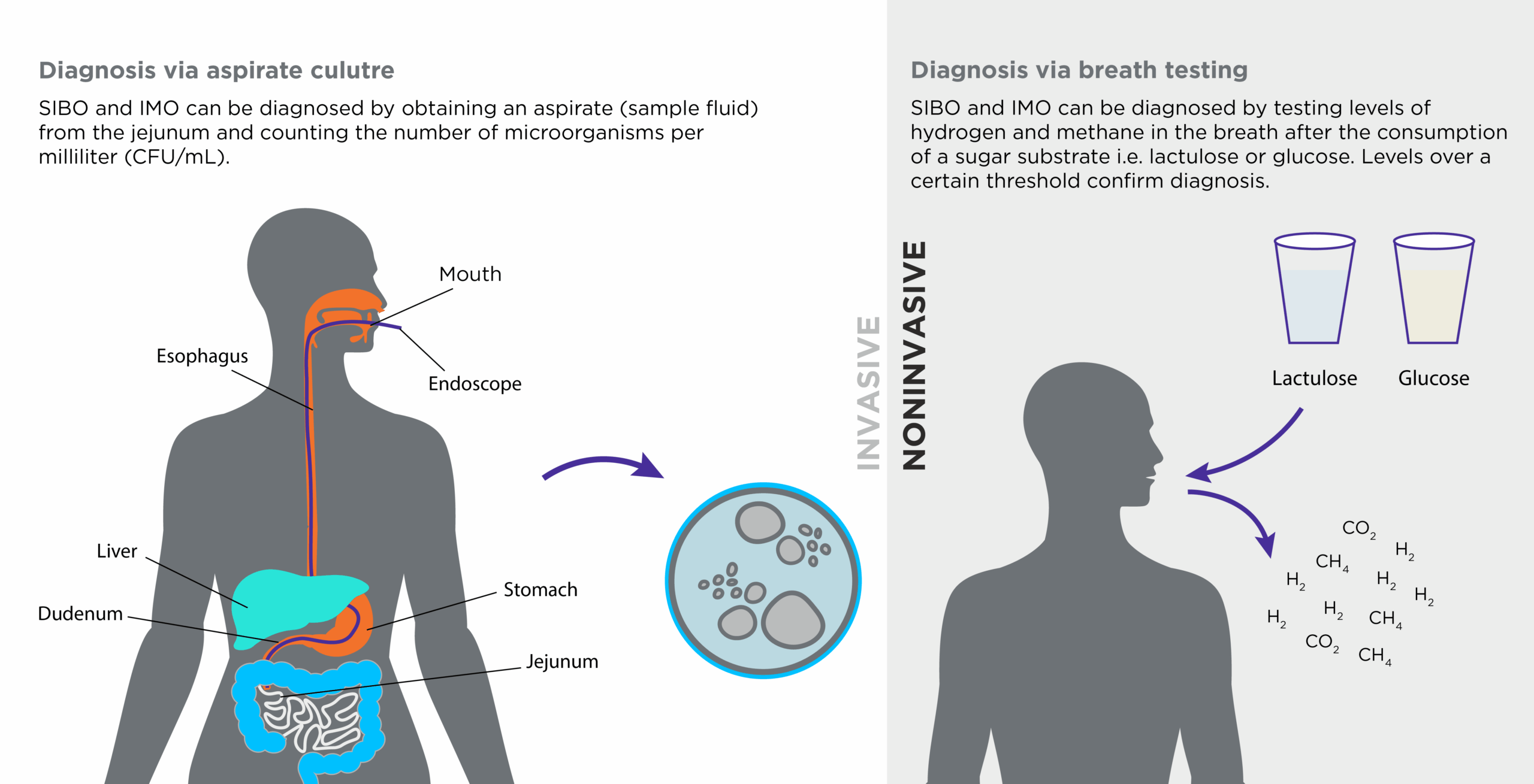
Figure 1: Invasive and non-invasive diagnostic tests for small bowel microbial overgrowth
When testing for SIBO and IMO, breath tests are commonly used due to their non-invasive nature and ease of use (see figure 1). Subjects must first ingest a sugar substrate, commonly glucose or lactulose, which enables microbial fermentation and gas production. Breath is then captured at various timepoints post ingestion and analysed for the levels of hydrogen and methane, which form a basis for diagnosis. Another diagnostic method is testing jejunum aspirate culture, an invasive test involving fluid extraction (endoscopy) from the small intestine (6). Although this method is considered the gold standard for SIBO diagnosis, there is no currently accepted best practice approach in terms of testing for gut dysbiosis in patients with liver disease.
In a meta-analysis of SIBO in cirrhosis, the authors found a SIBO prevalence of 40.8% in cirrhosis patients and 10.7% in controls (5). Yet, different testing methods found slight variation, with the highest prevalence of SIBO found using the aspirate culture, followed by lactulose tests and the lowest percentage of SIBO in cirrhosis patients was found when using glucose tests (see table below).
| Test Method | % of SIBO occurrence in cirrhosis patients | % of SIBO occurrence in healthy controls | Number of studies involved |
| Jejunum aspirate culture |
48% |
10.6% | 5 |
| Lactulose breath test |
52.7% |
17.5% |
4 |
|
Glucose breath test |
35.9% | 6.4% |
14 |
Table 1: Comparison of SIBO test results in cirrhosis patients, differentiated by test method
In comparison to using glucose, lactulose breath tests may have been more effective in detecting gut dysbiosis because this substrate is a synthetic, non-absorbable sugar, allowing it to reach any microbial overgrowth in the small intestine. Since cirrhosis is often associated with delayed transit time, glucose may be fully absorbed before it reaches the area of bacterial overgrowth in a patient with slowed motility, leading to a false-negative result. Lactulose, being non-absorbable, overcomes this limitation.
In another review of studies, diagnostic methods for SIBO in patients with Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) were explored (7). MASLD is a condition that can progress to cirrhosis. The analysis showed the highest odds ratio (OR) for the association between SIBO and MASLD was with aspirate culture (OR = 8.75), followed by the lactulose breath test (OR = 3.51), and then the glucose breath test (OR = 2.34). This suggests that while breath tests remain valuable, direct culture is more strongly correlated with the condition.
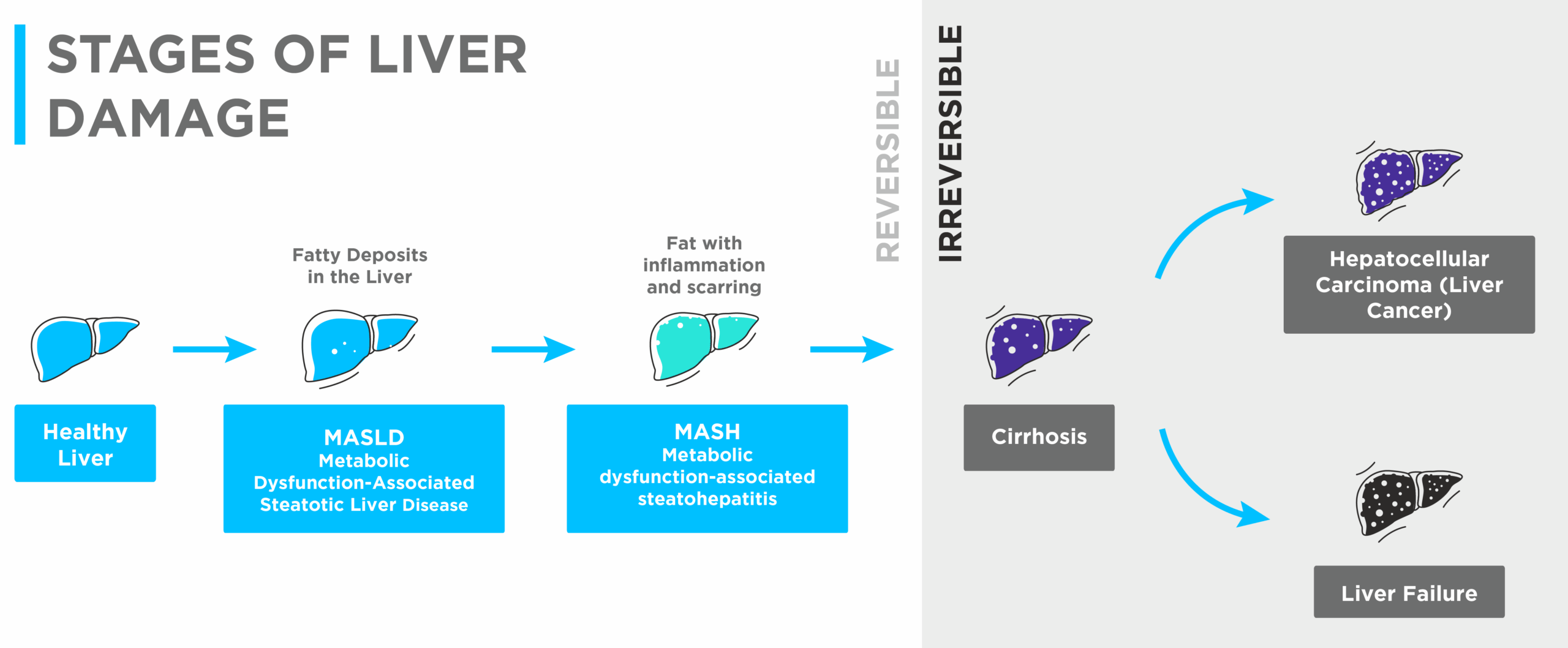
Figure 2: Illustration of the changing levels of liver damage severity
Leaky Gut and the Liver
The probability of developing SIBO has been shown to increase with liver damage severity (see figure 2 above). A meta-analysis found that odds ratios for MASLD, MASH (metabolic dysfunction-associated steatohepatitis), and fibrosis were 2.59, 4.35 and 4.73 respectively, meaning that SIBO is more likely to co-occur in increasingly severe cases of liver disease (7).
While many factors may contribute to liver disease, as shown in figure 3, a potential mechanism behind the connection between SIBO and liver disease is increased intestinal permeability, also known as leaky gut. Though many factors contribute to leaky gut, gut microbiome dysbiosis is known to damage the intestinal barrier (8). When chronically disrupted, waste products, toxins and pathogens can leak through gaps between cells, or through the cells themselves, and enter the bloodstream (9).
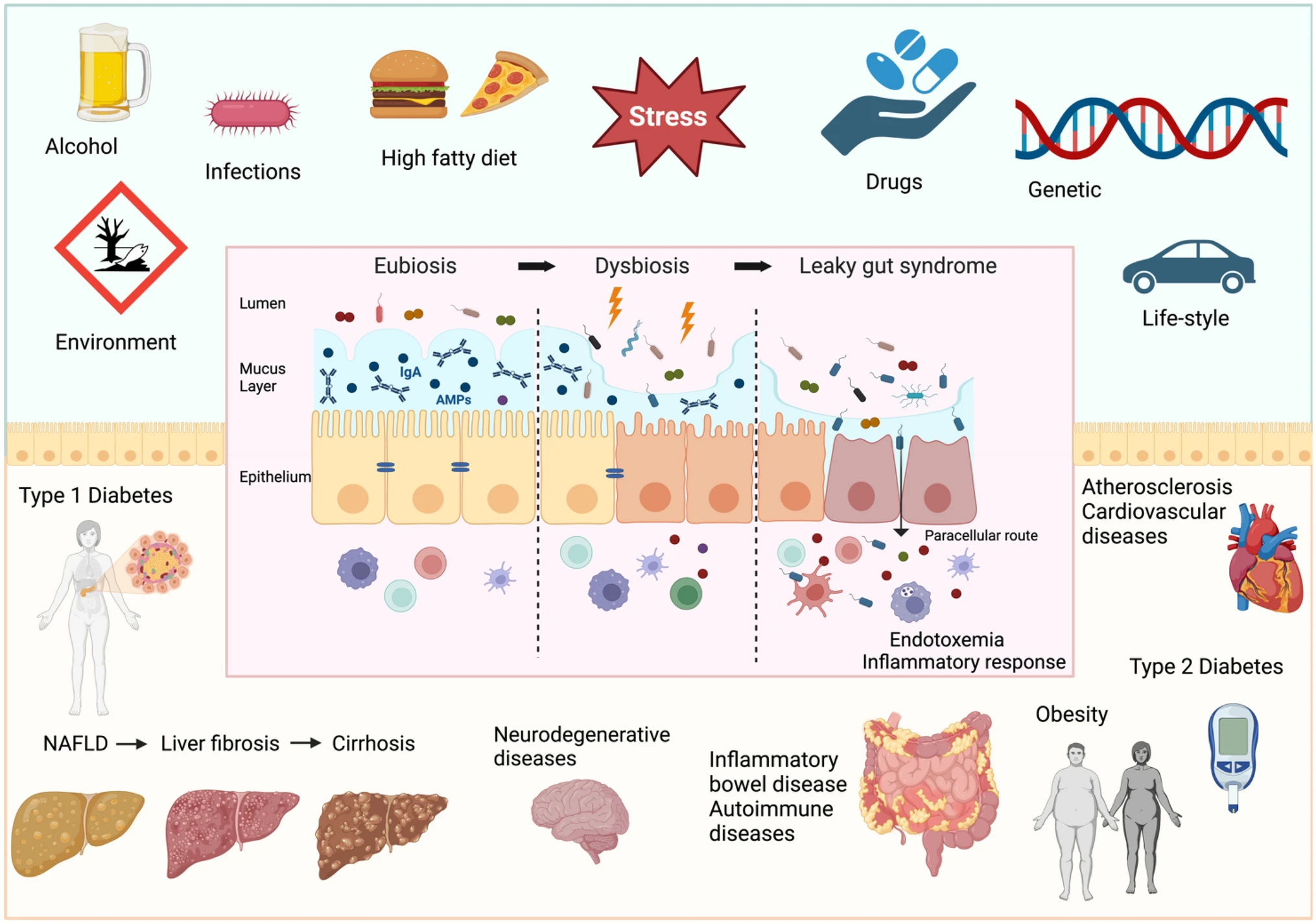
Figure 3: ‘Factors determining intestinal barrier impairment and consequent systemic diseases’, sourced from Di Vincenzo et al 2023 (10).
When microbial endotoxins enter the bloodstream, they travel straight to the liver via the portal vein. In the liver, these toxins activate receptors that trigger the production of pro-inflammatory molecules which induce inflammation and insulin resistance. Liver cells are further stimulated to produce triglycerides (fats). Alongside this, hepatic stellate cells (HSCs) are activated to transform into myofibroblasts – a mechanism that contributes to liver scarring, a hallmark sign of liver fibrosis. This inflammatory cascade may also drive liver disease comorbidities such as type 2 diabetes, food sensitivities and neurological conditions (11).
The Role of Methanogens
Methane producing archaea have a disproportionately large impact on the gut despite their relatively small numbers (12). One key reason for this is that methanogens consume hydrogen, which is needed to generate butyrate, a short chain fatty acid (SCFA) (13). It is known to support the integrity of the intestinal barrier and has been found to be protective against MASLD by enhancing fat oxidation. This is because butyrate can help the body burn fat more efficiently, helping to reduce fat build-up in the liver and prevent the development and progression of MASLD (14).
While baseline populations of methanogens contribute to a balanced ecosystem, excessive methane production correlates with slower transit times and IMO, both of which are independently associated with “leaky gut” in clinical cohorts (15). Since leaky gut occurs in 17–65% of people with liver conditions, microbial overgrowth is a potential mechanism behind non-alcoholic liver conditions. This is further supported by a meta-analysis of studies that included intestinal permeability testing, finding a strong association between gut dysbiosis and MASLD (7).
The presence of methanogens can also interfere with host metabolism. Methanogens have been shown to influence choline and bile acid metabolism, which are critical for liver health (16). Yet, it is important to keep in mind the bidirectionality of such interactions. For instance, bile acid can inhibit methanogenesis by reducing the number of methanogens present (17). There are a variety of complex interactions at play, many of which require further research to be fully understood.
Overall, research indicates that both IMO and SIBO are not merely a sign of gut dysbiosis, but are linked to specific pathophysiological mechanisms, such as increased intestinal permeability and altered host metabolism, that actively drive the progression from a healthy liver to steatosis, inflammation, and ultimately cirrhosis.
How useful is breath testing for gut-liver axis research?
Hydrogen-methane breath tests provide a non-invasive, cost-effective method to phenotype gut dysbiosis in liver disease. While causality remains to be fully established, the strong associations between SIBO, IMO and liver pathology suggest their utility in the following ways:
- Screening: early identification of high-risk patients for MASLD progression or cirrhosis decompensation
- Therapeutic trials: targeted antibiotics or probiotics to restore balance, strengthen barrier integrity, and potentially halt liver inflammation
- Combined biomarker panels: integrating breath testing with serum or stool markers (e.g., LPS, SCFAs) for a multi-modal gut–liver health assessment.
At Owlstone Medical, we offer breath testing solutions that enable a deep dive into the gut-liver axis. Detect hydrogen and methane in human breath with the OMED Health Breath Analyzer, enabling convenient testing with immediate results anytime, and anywhere. To determine liver function, look no further than the LIBRA® Liver Function Test. Using the LIBRA® Oral Solution, you can assess both hepatic extraction and metabolic activity without the need for invasive biopsies. Simply send back your breath collection device after use and await the analytical results. If you are interested in researching the gut microbiome, liver function or both – do not hesitate to contact us for further information.
References:
- Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, et al. Microbiota in health and diseases. Sig Transduct Target Ther [Internet]. 2022 Apr 23 [cited 2025 Jun 26];7(1):135. doi: 10.1038/s41392-022-00974-4
- Kunkel D, Basseri RJ, Makhani MD, Chong K, Chang C, Pimentel M. Methane on Breath Testing Is Associated with Constipation: A Systematic Review and Meta-analysis. Dig Dis Sci [Internet]. 2011 Jun 1 [cited 2025 Jun 26];56(6):1612–8. https://doi.org/10.1007/s10620-011-1590-5
- Samuel BS, Hansen EE, Manchester JK, Coutinho PM, Henrissat B, Fulton R, et al. Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc Natl Acad Sci U S A. 2007 Jun 19;104(25):10643–8. https://doi.org/10.1073/pnas.0704189104
- Martini GA, Phear EA, Ruebner B, Sherlock S. The bacterial content of the small intestine in normal and cirrhotic subjects: relation to methionine toxicity. Clin Sci. 1957 Feb;16(1):35–51. PMID: 13414138
- Maslennikov R, Pavlov C, Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: systematic review and meta-analysis. Hepatol Int. 2018 Nov;12(6):567–76. doi: 10.1007/s12072-018-9898-2
- Tang S, Li J, Ma J, Li Y, Li Y, Wan J, et al. Comparison of jejunal aspirate culture and methane and hydrogen breath test in the diagnosis of small intestinal bacterial overgrowth. Ir J Med Sci [Internet]. 2024 Apr 1 [cited 2025 Jun 26];193(2):699–703. https://doi.org/10.1007/s11845-023-03527-y
- Wang Z, Tan W, Huang J, Li Q, Wang J, Su H, et al. Small intestinal bacterial overgrowth and metabolic dysfunction-associated steatotic liver disease. Front Nutr. 2024;11:1502151. doi: 10.3389/fnut.2024.1502151
- Mechlińska A, Frąckiewicz K, Gładyś-Cieszyńska K, Buczek D, Dziadziuszko R. Small intestinal bacterial overgrowth and intestinal methanogen overgrowth in gastrointestinal malignancies. Contemp Oncol (Pozn) [Internet]. 2025 [cited 2025 Jun 26];29(1):11–21. PMID: 40330452
- Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med [Internet]. 2024 Mar 1 [cited 2025 Jun 26];19(2):275–93. DOI: 10.1007/s11739-023-03374-w
- Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med [Internet]. 2024 Mar 1 [cited 2025 Jun 27];19(2):275–93. Available from: https://doi.org/10.1007/s11739-023-03374-w
- Leech B, Schloss J, Steel A. Association between increased intestinal permeability and disease: A systematic review. Advances in Integrative Medicine [Internet]. 2019 Mar 1 [cited 2025 Jun 26];6(1):23–34. doi: 10.1016/j.aimed.2018.08.003
- Zengin ÖD, Aydin S, Zengin ÖD, Aydin S. The Hidden Influence of Methanogens in the Gut Microbiota. In: Methanogens – Unique Prokaryotes [Internet]. IntechOpen; 2025 [cited 2025 Jun 26]. doi: 10.5772/intechopen.1008814
- Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, et al. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep [Internet]. 2017 Sep 13 [cited 2025 Jun 26];7(1):11450. doi: 10.1038/s41598-017-11734-8
- Pant K, Venugopal SK, Pisarello MJL, Gradilone SA. The Role of Gut Microbiome-Derived Short-Chain Fatty Acid Butyrate in Hepatobiliary Diseases. The American Journal of Pathology [Internet]. 2023 Oct 1 [cited 2025 Jun 26];193(10):1455–67. doi: 10.1016/j.ajpath.2023.06.007
- Kunkel D, Basseri RJ, Makhani MD, Chong K, Chang C, Pimentel M. Methane on breath testing is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci. 2011 Jun;56(6):1612–8. doi: 10.1007/s10620-011-1590-5
- Watkins AJ, Roussel EG, Webster G, Parkes RJ, Sass H. Choline and N,N-dimethylethanolamine as direct substrates for methanogens. Appl Environ Microbiol. 2012 Dec;78(23):8298–303. doi: 10.1128/AEM.01941-12
- Florin TH, Woods HJ. Inhibition of methanogenesis by human bile. Gut. 1995 Sep;37(3):418–21. doi: 10.1136/gut.37.3.418