The potential role of VOCs in detecting and treating lung cancer
Published on: 12 Sep 2022
For several decades, Lung cancer has remained the leading cause of cancer-related deaths, accounting for 12.9% of all new cancer cases in the United States. The patient outcomes and 5-year survival rates are dependent on the stage of disease at diagnosis [1]. Thus, early diagnosis is crucial to saving the lives of lung cancer patients. Unfortunately, there are significant shortcomings in the current screening methods for early lung cancer detection.
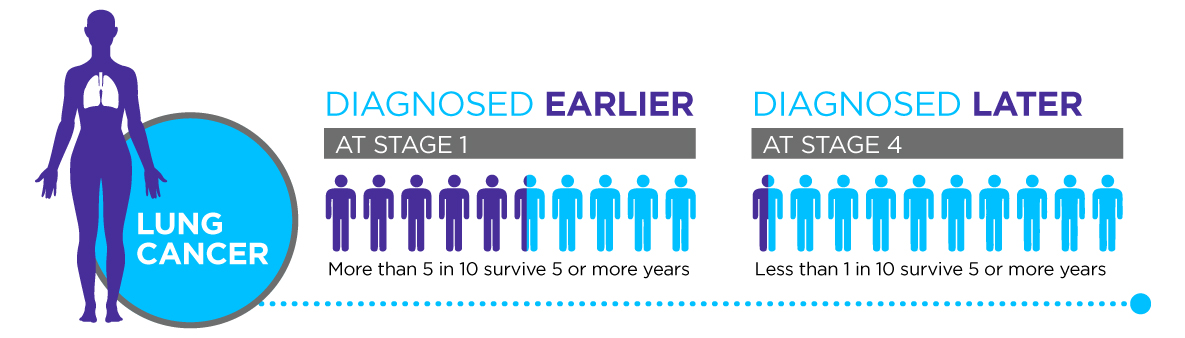
Limitations in current screening
Lung tumors may be present for several years before exhibiting clinical signs or symptoms, often going undetected in early stages. Lung cancer is therefore commonly diagnosed radiographically using computerized tomography (CT) imaging of once the disease has already progressed to later stages. Even at a late stage the patients’ symptoms will still be non-specific (they might include a cough, spitting blood, shortness of breath, and chest pain) and could relate to a number of respiratory diseases.
To mitigate the effects of high radiation exposure, low-dose CT (LDCT) scans are used in screening programs. Using LDCT, the National Lung Screening Trial (NLST) in the USA in 2011 enrolled 53,454 subjects and found a 20% reduction in lung cancer-specific mortality rate and a reduction of 6.7% in overall mortality rate [3]. The Center for Disease Control and Prevention recommends screening all smokers or ex-smokers aged 50 to 80 with a 20 pack-year smoking history in the past 15 years. Despite these strong pushes for lung cancer screening since 2013, most lung cancer patients are still diagnosed either after manifestation of late-stage symptoms or as an incidental finding after imaging.
To improve patient outcomes, it’s vital that lung cancer screening is offered to a wider population of at-risk patients. Currently only 27% of patients developing lung cancer in the USA would qualify for screening under the NLST entry criteria [4]. Despite the benefits of LDCT-based screening, there are also significant drawbacks that limit widespread adoption of the technique.
A key contributing factor is the high false positive rate of LDCT screening (23.7% false-positive rate in the NLST trial) which leads to both unnecessary costs and unnecessary burdensome treatments for patients [5]. There is a clear need for new lung cancer screening techniques, capable of detecting lung cancer earlier, more cheaply and with better discriminatory power.
Why use breath testing for lung cancer?
One of the early indicators that breath testing had potential utility in early-stage cancer diagnosis were studies in which dogs were able to distinguish the exhaled breath samples of patients with lung cancer from healthy controls [6].
Breath testing is much more suited to early-stage testing than an invasive tissue biopsy (a standard follow up to LDCT). Exhaled VOCs can relate directly to metabolism and how this changes distinctly during illness. Metabolic biomarkers offer high sensitivity as the effect of changes to genes or proteins, even when a tumor is very small, can be amplified in metabolism.
Breath sampling is also especially appropriate as a way to investigate respiratory illnesses due to breath’s proximity to the lungs and respiratory tract. It’s non-invasiveness compared to blood-sampling might also lead to higher compliance to improve lung cancer screening rates [2].
Check out our blog on why you should use breath.
We’re working to develop breath screening now
While the genomics of cancer tumors can vary extensively, these changes converge onto key metabolic pathways. The use of agents such as 18-FDG in PET scans illustrates how metabolic pathways can be targeted with an exogenous compound to provide high sensitivity cancer detection. At Owlstone Medical we’re investigating whether a similar approach can be harnessed in combination with breath sampling to enable earlier detection through our EVOC probe approach.
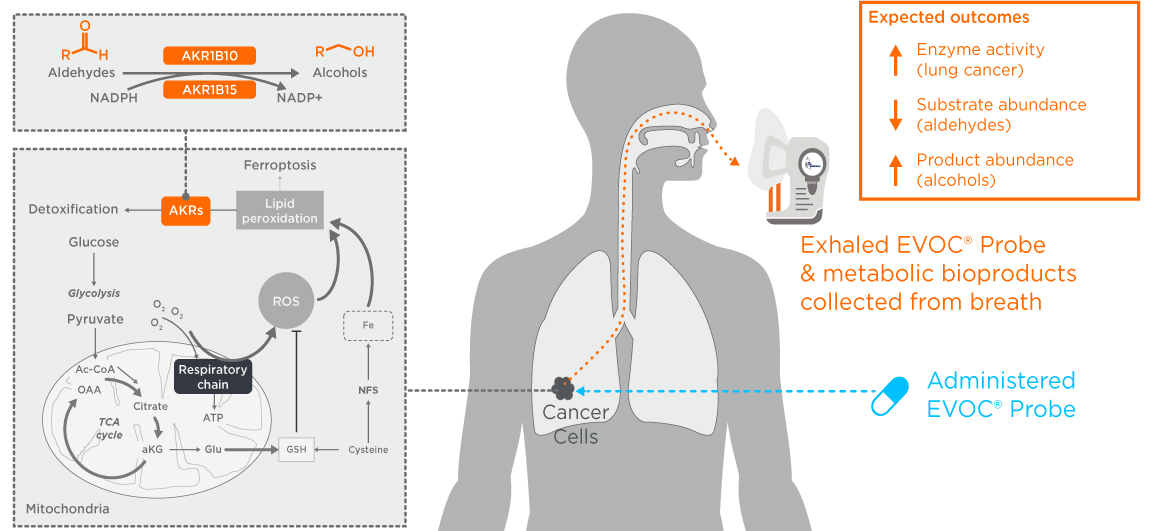
Human lung cancers increase expression of aldo-ketoreductase (AKR) enzymes to help process the excess aldehydes produced by oxidative stress. We conducted a successful in vitro study targeting AKRs using an exogenous volatile organic compound probe (EVOC® Probe). We’re now undertaking in vivo and ex vivo studies to see if this metabolic process can, as we predict, be monitored on breath using Breath Biopsy.
See our data Our clinical trial in the news
Breath testing could also allow precision treatment selection
Upon identification of a tumor, treatment options for lung cancer include surgery, radiation therapy, chemotherapy, and targeted therapies. Based on classification, lung cancer is broadly categorized into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). While cytotoxic chemotherapy has been the cornerstone of cancer treatment, precision medicine approaches based on identifying specific somatic mutations in tumors or other molecular features are now also in use. New approaches and new biomarkers are constantly being pursued in this area.
Biomarker based approaches are used to help select the most appropriate therapy for patients. These include running a biopsy through a targeted panel of tests to determine histologic subtype and assesses eligibility for targeted therapy or immunotherapy. NCCN guidelines include testing for biomarkers, including activating genetic mutations, for the planning of patient care [7]. Peled et al. used nineteen human NSCLC cancer cell-lines to demonstrate that tumors expressing some actionable biomarkers produce a unique VOC profile (potentially detectable on breath) [8]. The cell lines included tumor cells with EGFR, KRAS and ALK mutations and were reportedly shown to have different VOC profiles. This study yielded a classification success of 70% sensitivity, 100% specificity and 92% accuracy for distinguishing EGFR mutations from wildtype. The ALK mutations tests had slightly lower sensitivity (63%), while the KRAS mutation tests had higher sensitivity (93%) but lower specificity (78%) and accuracy (84%). Peled et al. suggest furthering the findings in this study by studying exhaled breath for accessible in-vivo VOCs.
Work with us to develop novel lung cancer diagnostics
We have created Breath Biopsy® OMNI® to be the most advanced solution for reliable global breath biomarker analysis. If you choose to work with us, to investigate another aspect of breath testing for lung cancer your work will be further supported by our extensive study design, management, and data interpretation expertise.
Developing a clinically useful test requires identification of the VOCs that can be linked to underlying biology and the specific disease of interest. Our Breath Biopsy Collection Station maximizes your chances of discovering robust breath biomarkers by ensuring optimal sample collection. Then Breath Biopsy OMNI uses TD-GC-MS with high resolution and high dynamic range, maximizing the number of VOCs that can be studied in your breath sample.
Find out more about OMNI Let’s chat
References
- Bade, Brett C.; Dela Cruz, Charles S. (2020). Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clinics in Chest Medicine, 41(1), 1–24.DOI: 10.1016/j.ccm.2019.10.001
- Li, Z., Shu, J., Yang, B., Zhang, Z., Huang, J., & Chen, Y. (2020). Emerging non-invasive detection methodologies for lung cancer. Oncol Lett, 19(5), 3389-3399. DOI: 10.3892/ol.2020.11460
- Jones, G. S., & Baldwin, D. R. (2018). Recent advances in the management of lung cancer. Clin Med (Lond), 18(Suppl 2), s41-s46. DOI: 10.7861/clinmedicine.18-2-s41
- Rogers, T. K. (2019). Minimising diagnostic delay in lung cancer. Thorax, 74(4), 319-320. DOI: 10.1136/thoraxjnl-2018-212927
- Aberle, D. R., Adams, A. M., Berg, C. D., Black, W. C., Clapp, J. D., Fagerstrom, R. M., Gareen, I. F., Gatsonis, C., Marcus, P. M., & Sicks, J. D. (2011). Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med, 365(5), 395-409. DOI: 10.1056/NEJMoa1102873
- McCulloch, M., Jezierski, T., Broffman, M., Hubbard, A., Turner, K., & Janecki, T. (2006). Diagnostic accuracy of canine scent detection in early- and late-stage lung and breast cancers. Integr Cancer Ther, 5(1), 30-39. DOI: 10.1177/1534735405285096
- Joshi, E., Nanayakkara, B., Barnes, D. J., & Troy, L. K. (2020). Precision Medicine in Lung Cancer. European Medical Journal Oncology. DOI: 10.33590/emjoncol/19-00145
- Peled, N., Barash, O., Tisch, U., Ionescu, R., Broza, Y. Y., Ilouze, M., Mattei, J., Bunn, P. A., Jr., Hirsch, F. R., & Haick, H. (2013). Volatile fingerprints of cancer specific genetic mutations. Nanomedicine, 9(6), 758-766. DOI: 10.1016/j.nano.2013.01.008
Catch up on the presentations from the Breath Biopsy Conference 2024
