The Intersection between Cancer and the Microbiome
Published on: 2 May 2023
Cancer is one of the most important global health issues. According to the World Health Organization, all cancers were collectively responsible for an estimated 10 million deaths in 2020 (1), placing it as one of the leading causes of death.
The discovery that the human body is colonized by microbial species of bacteria, fungi, and archaea has transformed how we think about the processes behind health and disease. This community of microbes – collectively referred to as the microbiota, or the microbiome – has been linked to the pathophysiology of many diseases including cancer, and therefore opens the door to a new category of potential biomarkers.
The gut microbiota has a well-established role in regulating the immune system, and the composition of the microbial community here can impact the efficacy of a class of anti-cancer therapy that utilizes the immune system, known as immune checkpoint inhibitors (3). This highlights one important mechanism through which the microbiome can interplay with the body and impact cancer development, and treatment. Many microbial compounds that could be used as biomarkers also happen to be volatile, and therefore could be measured in exhaled breath. Therefore, breath analysis could prove a vital platform for investigating the clinically relevant links between the microbiome and cancer.
Colorectal cancer (CRC) is currently the third leading cause of cancer death, with an increasing incidence rate (2). Using CRC as a model system, this blog post will explore some of the underlying principles of how the microbiome can interact with the body and contribute to cancer, and finally how breath can offer an innovative new platform for biomarker research in this area.
The Large Intestine is the Stage for many Host-Microbe Interactions.
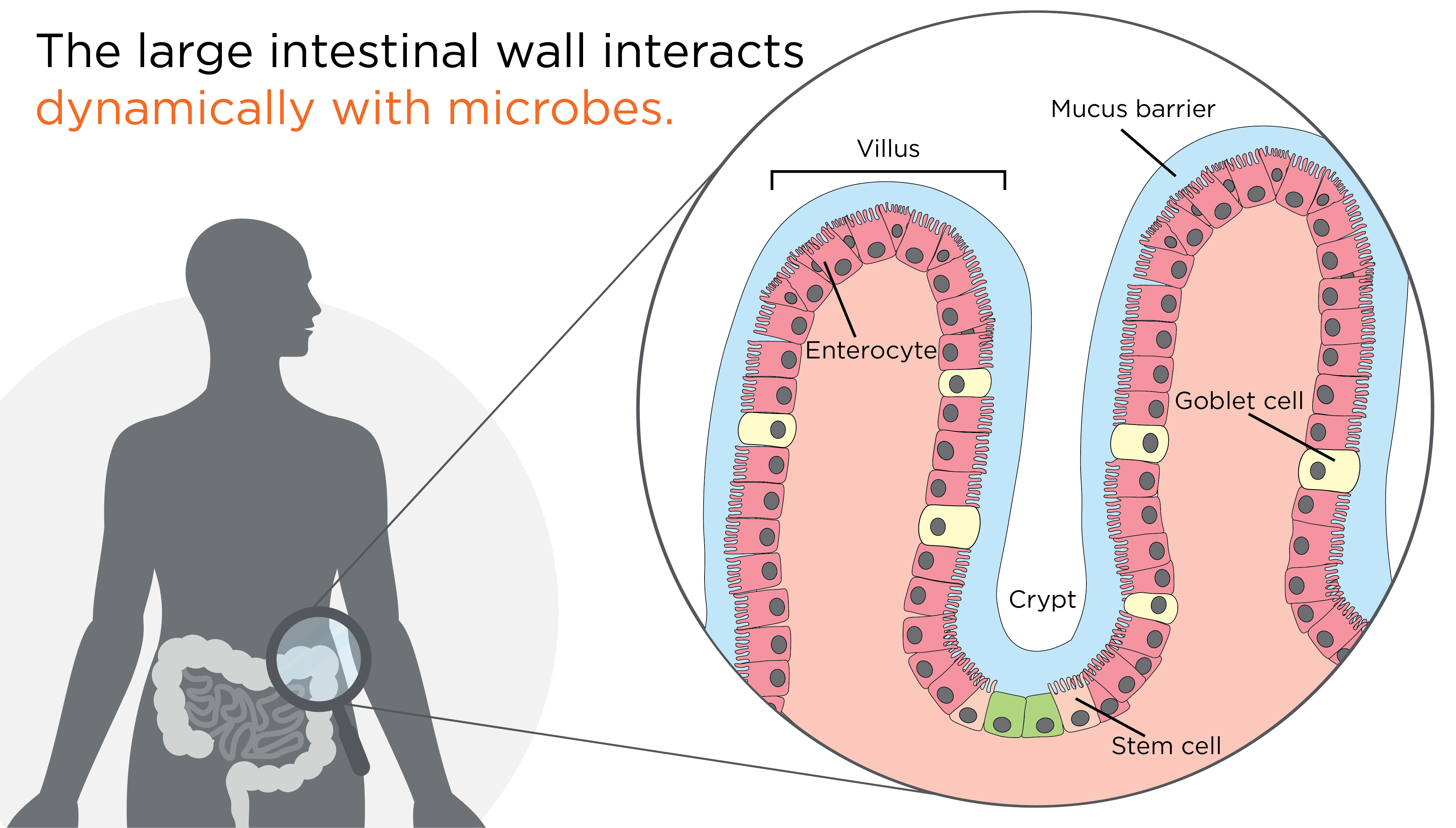
The wall of the large intestine is made up of distinct layers of tissues, all with their own specific cell types. Enterocytes are the most common cell type in the outer (epithelial) layer, with the primary role of absorbing nutrients.
Usually, gut microbes are prevented from directly contacting the cells of the intestinal epithelial layer by a thin layer of mucus secreted by goblet cells, another cell type in the epithelial layer (4). This mucus barrier is extremely important, it allows nutrients to flow into the enterocytes as well as towards the symbiotic microbiota to maintain their balance, protecting the intestine from mechanical stress, and physically separating the epithelial cells from the gut microbiota to buffer against potentially harmful secreted compounds.
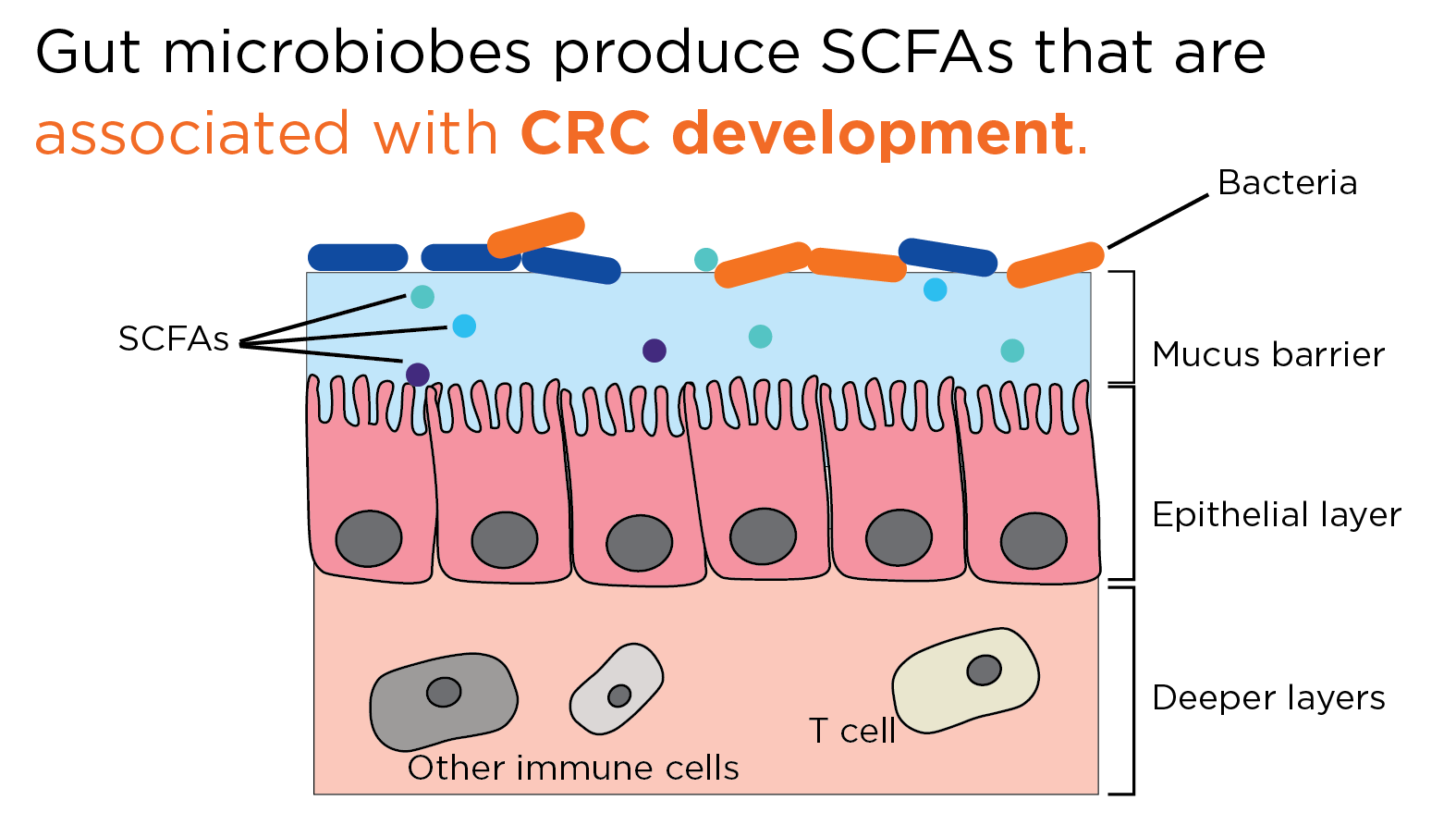
If the integrity of the mucus barrier is disrupted, this can allow for direct contact between microbes and the intestinal cells, as well as contact between their various secreted metabolites to reach the surface cells, and potentially penetrate deeper into the intestinal tissue. Upon disruption of the intestinal epithelial barrier, compounds produced via the microbial metabolic or infectious pathways can interfere with cellular mechanisms, including the control of cellular proliferation, inflammation, and further disruption of the epithelial barrier. Together, this creates an environment sufficient for tumor development (5,6).
Under these conditions, normal cells of the intestinal tract can be pushed toward a cancerous state, and there is a large body of clinical evidence that the gut microbiome has a strong association with the development of CRC (5). Perturbations of the microbial community into dysbiosis can have both direct and indirect impacts on cancer development, and to follow how the microbiome can contribute to the development of CRC, the microbially-derived short chain fatty acids (SCFAs) can be used as an example.
SCFAs and Cancer.
Bacteria in the gut produce many SCFAs such as acetate, butyrate, and propionate, all three of which have been associated with the suppression of inflammation in the gut, and CRC development (7) One of the most common symbiotic bacterial phyla that colonize the intestinal tract is Firmicutes, and alongside Bacteroides, make up 95% of the microbial colonizers of the gut (9,10). These bacterial phyla are major producers of acetate and propionate, and the reduction of Firmicutes bacteria in the gut with increasing age is associated with a shift in the microbiota towards a more inflammatory state, and tumorigenic environment (5).
Acetate makes up the majority of SCFAs produced in the colon, and butyrate serves as a primary source of energy for enterocytes, lower levels of butyrate and butyrate-producing bacteria have been continually linked to CRC incidence (6). Both butyrate and propionate have been associated with essential regulation of T cell function, which helps to control levels of inflammation in the large intestine. There are many mechanisms through which butyrate in particular can impart an anti-cancer effect, including inducing apoptosis in CRC cells, epigenetic modulation through inhibiting histone deacetylases, serving as an anti-inflammatory signal, reduction of transit time, and contributing to the maintenance of the intestinal epithelial barrier (6,8).
Altogether, this points to a fundamental mechanism through which SCFA levels produced by the gut are linked to CRC risk and development. Therefore SCFAs, and other microbially-produced compounds could serve as important biomarkers for assessing an individual’s predisposing factors for cancer development and monitoring cancer progression.
SCFAs are Volatile, and many are Detectable in Exhaled Breath.
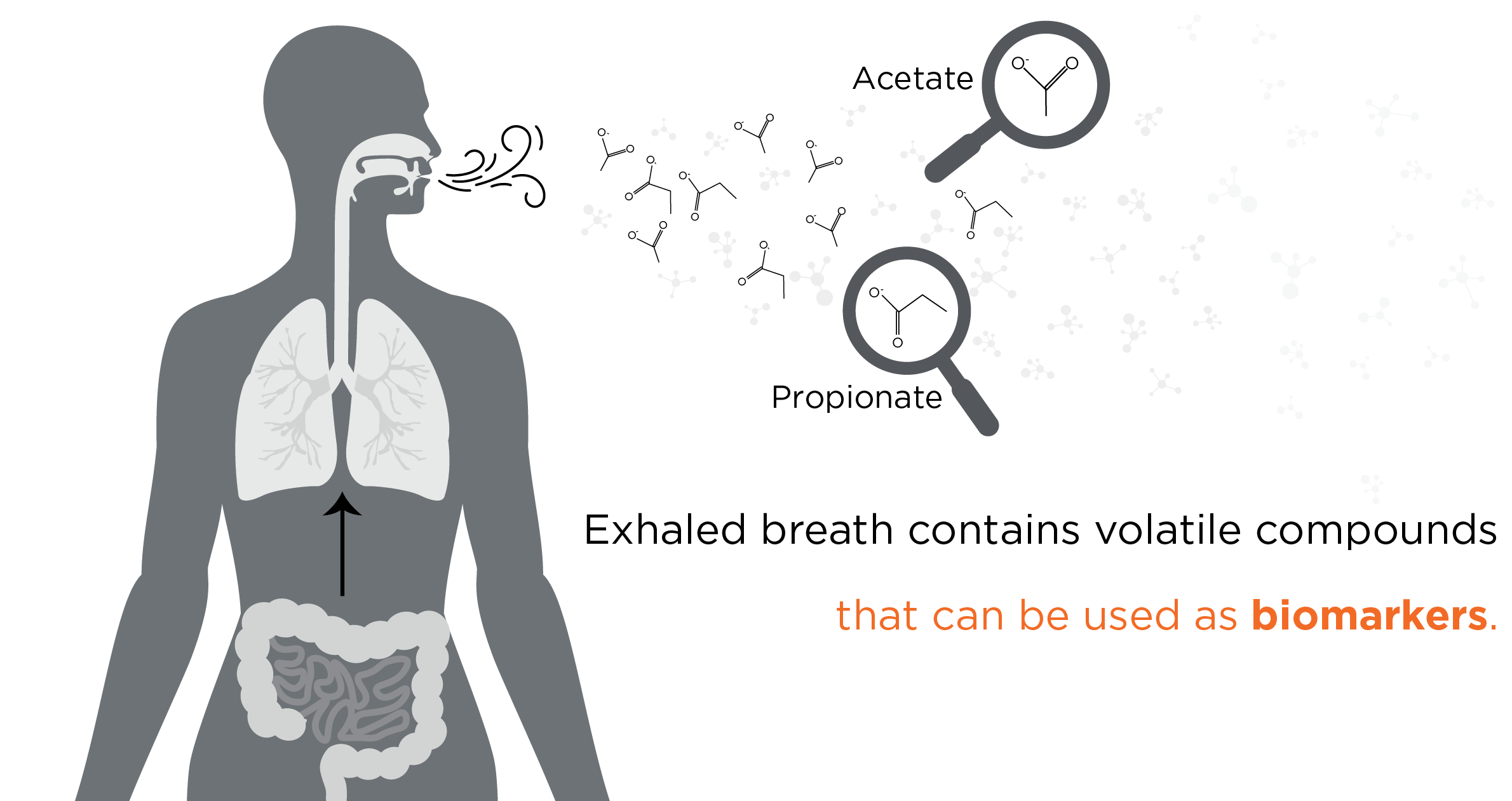
SCFAs are a group of volatile compounds, many of which are readily detectable in exhaled breath and included in our VOC Atlas, including acetate, butyrate, and propionate. There are also other SCFAs in our VOC Atlas, such as isobutyric acid, 2-methylbutanoic acid, and isovaleric acid. Volatile SCFAs produced in the gut are readily able to enter the bloodstream, which circulates throughout the body and can enter the air in the lungs via the network of blood vessels surrounding the alveolar membrane. This air enriched for volatile compounds from throughout the body is then exhaled. Therefore, endogenously produced compounds detected in the breath can provide a window into microbial metabolism ongoing in the gastrointestinal tract.
Based on the production of hydrogen gas by gut microbes, the time between the production of a volatile compound in the gut to measurable detection in the breath has been measured to be five minutes or less (11). As breath is a virtually inexhaustible resource, large volumes can be collected at frequent intervals, and compounds can also be pre-concentrated prior to analysis. These factors combined make breath analysis capable of almost real-time longitudinal analysis of volatile metabolites, and SCFAs can offer key biomarkers for CRC for clinical studies. There are many more volatile compounds, including those that are microbially produced in our VOC Atlas, many of which have been associated with cancer.
We are the world leader in Breath Biopsy for early detection and precision medicine, and we are here to help you to include breath analysis in your research. Our Breath Biopsy OMNI provides everything you might need to start using breath analysis, as well as expert advice from our team of scientists. With this solution, you will now be able to cross-check your data against our VOC Atlas to better understand how individual VOCs behave in a healthy population and confidentially validate your cancer or microbiome biomarkers.
Our in vitro headspace sampling can help you to identify VOCs produced by your laboratory samples such as urine, fecal matter, and blood, and see whether biomarkers that you are interested in could also be analyzed using breath analysis.
If you have any questions or would like more information, please do not hesitate to contact us to discuss investigating breath VOC biomarkers for cancer, the microbiome, or other diseases of interest.
References
- Cancer [Internet]. [cited 2023 Apr 19]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
- Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89–103. DOI: 10.5114/pg.2018.81072
- Li X, Zhang S, Guo G, Han J, Yu J. Gut microbiome in modulating immune checkpoint inhibitors. EBioMedicine. 2022;82:104163. DOI: 10.1016/j.ebiom.2022.104163
- Coleman OI, Haller D. Microbe–Mucus Interface in the Pathogenesis of Colorectal Cancer. Cancers (Basel). 2021 Feb 4;13(4):616. DOI: 10.3390/cancers13040616
- Raskov H, Burcharth J, Pommergaard HC. Linking Gut Microbiota to Colorectal Cancer. J Cancer. 2017 Sep 20;8(17):3378–95. DOI: 10.7150/jca.20497
- Mirzaei R, Afaghi A, Babakhani S, Sohrabi MR, Hosseini-Fard SR, Babolhavaeji K, et al. Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomedicine & Pharmacotherapy. 2021 Jul 1;139:111619. DOI: 10.1016/j.biopha.2021.111619
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014 Oct;12(10):661–72. DOI: 10.1038/nrmicro3344
- Han A, Bennett N, Ahmed B, Whelan J, Donohoe DR. Butyrate decreases its own oxidation in colorectal cancer cells through inhibition of histone deacetylases. Oncotarget. 2018 Jun 5;9(43):27280–92. DOI: 10.18632/oncotarget.25546
- Structure, Function and Diversity of the Healthy Human Microbiome. Nature. 2012 Jun 13;486(7402):207–14. DOI: 10.1038/nature11234
- Allaband C, McDonald D, Vázquez-Baeza Y, Minich JJ, Tripathi A, Brenner DA, et al. Microbiome 101: Studying, Analyzing, and Interpreting Gut Microbiome Data for Clinicians. Clin Gastroenterol Hepatol. 2019 Jan;17(2):218–30. DOI: 10.1016/j.cgh.2018.09.017
- Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011 Mar;60(3):334–40. DOI: 10.1136/gut.2009.205476