Building the Blueprint of Breath Biomarkers through Standardization – Highlights from the Breath Biopsy Conference 2024
Published on: 22 May 2025
Innovations in biomarker technology are transforming healthcare, enabling earlier detection of disease, improved treatment guidance, and real-time monitoring. Breath biomarkers are gaining attention due to their non-invasive collection, whole-body insight, and potential for at-home testing.
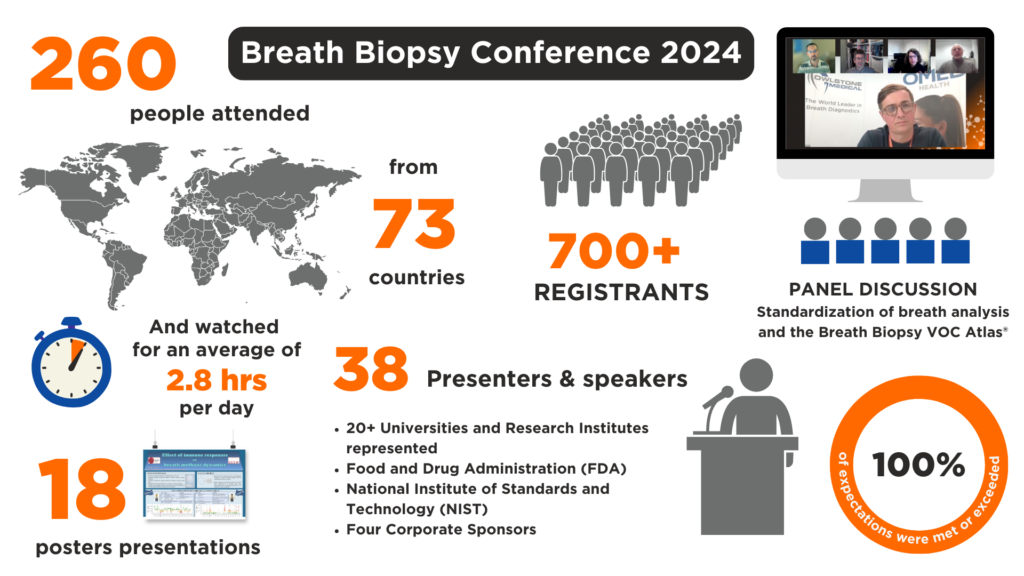
In November 2024, the seventh annual Breath Biopsy Conference brought researchers together from around the world to explore the latest in breath analysis.
Improving Data Reproducibility in Breath Research
One of the major themes at the event was the need for greater standardization in breath analysis to improve data reproducibility across studies. Inconsistent methodologies have made it difficult to compare results or validate findings, limiting progress in translating breath biomarkers into clinical practice.
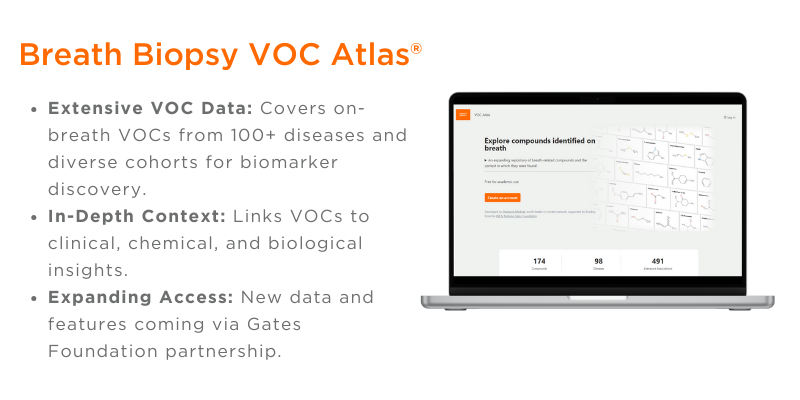
To help overcome this, Dr. Julia Greenwood at Owlstone Medical introduced the Breath Biopsy VOC Atlas®, a curated database of validated on-breath VOCs. This resource supports more consistent, high-quality research by providing chemical standards-based identification and quantitative data on VOC concentrations in exhaled breath. Public access to the Atlas is now available free of charge.
Government institutions are increasingly recognizing the importance of standardization in breath analysis and are now actively working to provide regulatory science tools (RST) to ensure high-quality breath analysis and VOC identification, and therefore improve the quality of data going into regulatory submissions. Two projects presented at the conference are helping to lay the groundwork for these tools. Dr Eric Miller at the FDA’s Office of Science and Engineering Laboratories (OSEL), shared work on developing an RST to enable the confident identification of compounds in the breath matrix. To streamline the identification process and establish criteria for interlaboratory consistency, 122 reference standards were selected and procured. Following on from this Dr Omar Rivera, also from the FDA, introduced the concept of a ‘Chemical Universe’ – a way of assessing the effectiveness of analytical methods in detecting all relevant chemicals, addressing the problem of inconsistent biomarker identification across studies. His team is already applying it in a tuberculosis study with Owlstone and the Gates Foundation.
Together, these efforts are laying a stronger foundation for breath research, making it easier for scientists to build on each other’s work. With tools like the VOC Atlas and new regulatory frameworks in development, standardization is becoming more than just a goal, but a norm.
Advancing Breath Research Methods
Technological innovations were another focus at the conference. Dr Helen Martin at Markes International, a loyal sponsor of the Breath Biopsy Conference, showcased instruments that streamline sample processing, like dual thermal desorption systems and tools for evenly distributing standards across multiple tubes—key to ensuring data quality when handling high sample volumes.
Dr. Flavio Franchina at University of Ferrara, on behalf of LECO, emphasized the value of harmonized methods for comparing results across breath, in vitro, and ex vivo studies.
On the innovation front, Dr. Alexandra Martin (Owlstone) presented advances in EVOC® probe technology—synthetic compounds designed to release detectable signals in breath when metabolized by disease-related enzymes. She shared how new methods have boosted sensitivity and reduced background noise, supporting EVOC use in both animal and human studies. Complementing this, Dr. Giuseppe Ferrandino (Owlstone) also showed how EVOCs could distinguish patients with liver cirrhosis from healthy controls based on metabolic differences observed in exhaled breath.
Biomarker Discovery: Exploring VOC Origins
Understanding the origins and significance of VOCs, especially the role of microbes and what their biochemical features tell us about health and disease, remains central to breath research.
Dr. Andrew Kau (Washington University) presented compelling evidence that breath VOCs reflect the complex activity of gut microbes. His innovative studies with children and gnotobiotic mice linked specific bacteria to distinctive breath VOC profiles.
In addition to the human microbiome, pathogenic microbes responsible for infectious diseases can also influence exhaled breath VOC profiles. Dr. Andres Floto (University of Cambridge) highlighted breath VOCs as promising markers for tracking cystic fibrosis exacerbations, offering new hope for better monitoring of infection and inflammation in vulnerable patients.
The plenary speaker, Dr. Oleg Shchelochkov presented groundbreaking findings on propionic acidemia, a rare metabolic disorder. His work identified a specific VOC, 3-pentanone, which was dramatically elevated in affected individuals and closely tied to underlying biochemical pathways and gut microbiome activity.
Dr. Yuta Matsuoka (Kyoto University) took us deep into the world of ferroptosis—a unique form of cell death, driven by iron-dependent lipid peroxidation—and revealed how breath VOCs like 1-octen-3-ol and 2-pentylfuran can serve as non-invasive markers for liver diseases, including metabolic-associated steatotic liver disease (MASLD).
Moving Towards Point-of-Care Diagnostics
Alongside biomarker discoveries, the conference explored exciting developments in portable breath analysis technologies designed for real-world healthcare applications. Dr. Daniela Polag (Heidelberg University) challenged traditional beliefs about methane production in breath, revealing dynamic patterns linked not only to microbes but also to oxidative stress and immune function. Leading onto this Dr. Joshua Bates and Dr. Riccardo Avvisati (Owlstone) introduced the OMED Health® Breath Analyzer, a compact, user-friendly device validated to accurately measure hydrogen and methane levels in breath. This data can be made immediately available to healthcare providers, enabling more comprehensive and personalized patient care.
Affordable, high-sensitivity breath tests are in high demand for infectious diseases like malaria and tuberculosis. Dr. Audrey Odom John (Children’s Hospital of Philadelphia) presented promising breath VOC biomarkers for malaria diagnosis, validated in studies across Malawi. Supported by the Bill & Melinda Gates Foundation, her research aims to develop non-invasive breath tests that could transform malaria detection, including for asymptomatic carriers.
In collaboration with the Gates Foundation, the National Institute of Standards and Technology (NIST) is focusing on advancing point-of-care devices. Dr. Kavita M. Jeerage (NIST) highlighted her team’s work on developing and delivering matrix-matched breath surrogates—engineered gas mixtures that mimic human breath to help calibrate and improve breath sensor technologies. This innovation is key to ensuring accuracy in emerging diagnostic devices for diseases like malaria and tuberculosis.
Ask the Expert: Standardization in Breath Analysis and Building a VOC Atlas
Each year, the Ask the Expert session is popular with attendees. The session offers the opportunity to hear the thoughts of selected experts, with four joining the CEO and Co-Founder of Owlstone Medical Billy Boyle. Dr. James Covington from the University of Warwick, Dr. Brooke Kaiser from the Pacific Northwest National Laboratory, Dr. Iain Robert White from the University of Nova Gorica, and Dr. Ben de Lacy Costello from the University of the West of England Bristol for the discussion on ‘Standardization in breath analysis and building a VOC Atlas.’
They tackled this critical topic by sharing insights on the biggest challenges and next steps for the field.
Catch the full session on demand here.