InTERCEPT: Early Diagnosis of Colorectal Cancer
The early detection of colorectal cancer aiming to save thousands of lives every year
OVERVIEW
Early detection of cancer saves lives
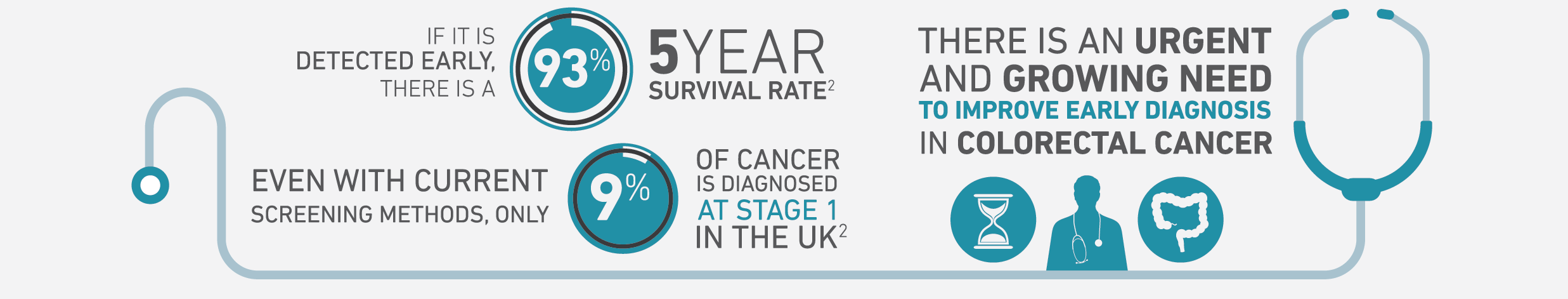
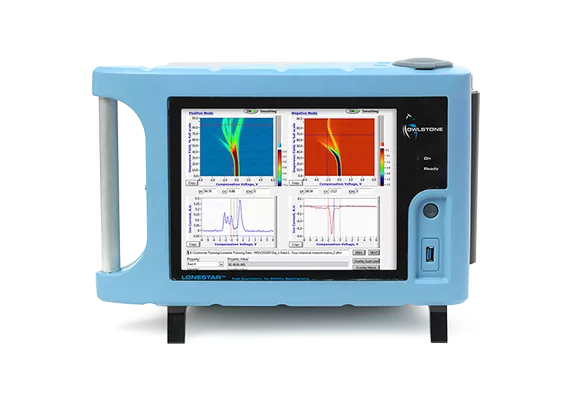
Pilot studies using Owlstone’s Lonestar® FAIMS platform technology have shown promising results. A study of volatile organic compounds in urine showed a sensitivity of 88% for colorectal cancer and 62% for advanced adenomas, a pre-cancerous stage of colorectal cancer. This is a substantial increase in the rate of detection compared with the current tests based on detecting blood in feces.
We believe that our screening test has the potential to save thousands of lives and billions of pounds in healthcare costs over the next 10 years.
In collaboration with Warwick University and the University Hospital Coventry and Warwickshire NHS Trust, Owlstone is running a 1,400 patient trial for colorectal cancer screening using breath and urine samples.
TAKING PART
Taking part in the clinical study
To be able to take part in this study the patient must be under clinical suspicion of having colorectal cancer, be aged 18 or older and agree to provide written consent to taking part in the clinical study. Patients who attend the UHCW hospital may be invited to take part; it is not possible to volunteer for this study.
You don’t have to take part and you can withdraw your consent at any time. Data is stored securely at Owlstone Medical and its researchers do not have access to any information which could be used to identify you.

What to expect if you are selected
If you are selected and agree to take part a nurse will explain the study to you and answer any of your questions. If you are happy to take part the nurse will fill in a questionnaire with information about you and your health and will take a sample of breath using Owlstone Medical’s ReCIVA breath sampler and ask you to provide a mid-stream urine sample. These samples will be analyzed by Owlstone Medical in its Cambridge Laboratory. These results, along with other information that we get from your doctor such as whether the tumour is cancer, will be used to develop a new test to screen for colorectal cancer.
STUDY PROTOCOL
Summary of the protocol
- DESIGN
The study is a single centre, prospective cohort study of up to 1,000 patients. Clinical outcomes will be monitored at 6 and 12 months
- PRIMARY OUTCOME
The primary aim is to validate the accuracy of VOC analysis for colorectal cancer and advanced adenoma detection in both breath and urine
- PATIENT ELIGIBILITY
Aged 18 or older, who have been referred under a clinical suspicion of having colorectal cancer to University Hospital Coventry and Warwickshire.
- WHAT TO EXPECT
After a briefing and agreeing to take part a nurse will take a sample of breath (using ReCIVA) and a urine sample
- BREATH ANALYSIS
Breath samples will be analysed using Owlstone’s FAIMS platform and GC-MS, urine samples using LC-UltraFAIMS-MS platform
- DATA ANALYSIS
The diagnostic algorithm will be developed on a random selection of two thirds of the study population, the training set.
CONSORTIUM
The people behind the clinical trial
The Principal Investigator for the InTERCEPT trial is Professor Ramesh Arasaradnam from Warwick University and Consultant Gastroenterologist at the University Hospitals Coventry and Warwickshire. Ramesh graduated from Queen’s and completed specialty training in Sheffield and doctoral research at Newcastle.
His clinical and research interests are in gut physiology, nutrition, inflammatory and cancer biology and he has several publications.
The Owlstone Medical PI is Dr Marc van der Schee. Marc obtained his Medical degree at VU University Amsterdam and has extensive expertise in all aspects of developing and running clinical trials using volatile biomarkers for disease diagnosis, monitoring and phenotyping. Marc is responsible for all clinical applications at Owlstone Medical.