Investigating breath biomarkers of insulin resistance
A non-invasive way to identify patients at risk of developing type 2 diabetes
| Publication information: Khan, M. S. et al. Breath biomarkers of insulin resistance in pre‑diabetic Hispanic adolescents with obesity (2022) Sci Rep 12, 339. DOI: 10.1038/s41598-021-04072-3
Disease Area: Diabetes Application: Early detection, ongoing monitoring Sample medium: Breath Products: ReCIVA® Breath Sampler Analysis approach: GCxGC-MS Summary:
|
Without appropriate and timely intervention, the path towards receiving a diagnosis of type 2 diabetes normally begins when a patient develops insulin resistance (IR). IR is a condition in which cells cease to respond to insulin correctly. While it’s commonly associated with increasing age, amongst younger patients it’s often considered a result of obesity coupled with insufficient exercise. As much as a quarter of the world’s adult population is affected. If left untreated, diabetes can progress to advanced stages where metabolic imbalance can lead to effects including poor blood flow, impaired eyesight, and nerve damage.
If IR is diagnosed early enough then the metabolic dysfunction may be able to be reversed before blood glucose levels reach the levels that will result in a clinical diagnosis of type 2 diabetes. Currently diagnosis of IR requires multiple blood samples, each preceded by fasting, which can be burdensome on at-risk groups when regular monitoring is required. As a result, this can stop some people getting tested, reducing the number of cases that are caught early. Without intervention, 70% of those with IR will progress to diabetes within 15-20 years. There’s therefore a huge need for new clinically relevant diagnostic tools that allow at-risk individuals to be identified earlier so as to either completely prevent or slow the development of type 2 diabetes.
Evidence from previous studies has shown that some volatile organic compounds (VOCs) on exhaled breath can reflect with metabolic activity occurring inside the body. This suggests that it might be possible to find breath-based biomarkers indicative of metabolic dysregulation that could offer a non-invasive solution for diagnosis and ongoing monitoring of IR.
In their study, Khan et al. set out investigate the feasibility of using ReCIVA Breath Sampler to collect samples within the obese and severely obese target group – a high-risk group for developing IR – and to identify putative biomarkers which seem to correlate with the presence of IR.
Results
28 obese and severely obese adolescents with an average age of 15.46 provided breath samples, none of whom had yet developed type 2 diabetes as judged through the typical diagnosis route of a blood test and clinical assessment – all breath samples were collected successfully with an average sample time of just over 4 minutes. At the same time as the breath samples were collected, blood samples were also conducted. In line with current best practice, blood was then used to define three groups: adolescents without IR, whose breath samples would be used as controls; adolescents with IR; and borderline cases.
After GCxGC-MS analysis of the breath samples a random forest regression model was used to identify the most relevant molecular features. 7 of the most significant 10 VOCs were tentatively identified as limonene, decane-2,4,6-trimethyl, undecane, undecane-2,7-dimethyl, pentylbenzene, octamethyloctane, eicosane by comparison against NIST library analytical standards.
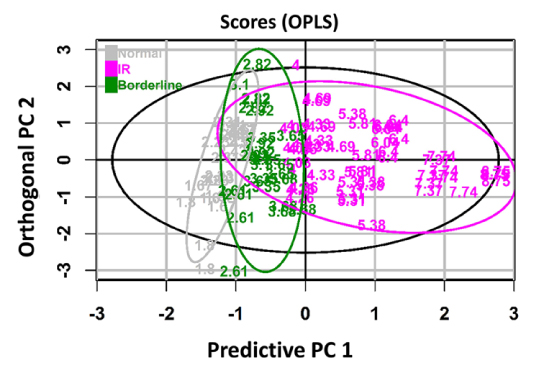
Figure 1: The score scatter plot showing the clustering of samples. The purple color indicates the IR group, the green color represents the borderline IR group and the normal group is presented as grey.
A breath IR model was developed based on those 10 features which showed strong correlation to existing clinical measures of glucose metabolism. Three of the 10 features (limonene, undecane, and undecane-2,7-dimethyl) were also found to be useful for differentiating IR and non-IR groups, the other 7 following the same trend but not to a statistically significant degree. Limonene in particular showed potential to also differentiate between borderline IR cases and controls, but not between IR cases and borderline cases.
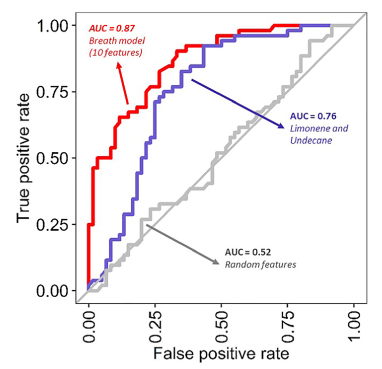
Figure 2: Diagnostics performance of the Breath-IR model. The receiver operating characters (ROC) curves using the random forest model between the IR group and the normal group of adolescents.
A breath IR model using all 10 compounds classified individuals with and without IR with an area under the receiver operating characteristic curve (AUC–ROC) of 0.87, with a sensitivity 73.1% and specificity of 81.7%.
Although 7 of the breath metabolites isolated did not perform well as differentiators between the groups on their own, collectively they nonetheless contributed positively to the Breath IR model. Khan et al. hypothesized that this might be because of some level of normal variability in levels of VOCs on breath between individuals. The breath IR model compares favorably with the currently accepted hemoglobin A1c method for diagnosing diabetes, suggesting that further investigation, particularly through testing with larger and more diverse cohorts, is warranted.
Further study is needed to ascertain how closely the metabolites can be identified with IR directly, or whether they are actually a consequence of early-stage liver disease. Both IR and liver disease are linked to obesity and associate with oxidative stress, which includes numerous metabolic shifts including increased production of reactive oxygen species.
The ReCIVA Breath Sampler used for sample collection in this study is just one part of the industry-leading breath sample collection and analysis platform, Breath Biopsy, that has been developed by Owlstone Medical in partnership with our academic and clinical collaborators.
Get in touch to find out how Breath Biopsy could support your own study with reproducible and reliable in vivo breath sample collection and breath biomarker analysis.
