Breath analysis for detecting cystic fibrosis and Pseudomonas exacerbations
Breath: A solution for ongoing monitoring of cystic fibrosis in the post-TRIKAFTA world
| Publication information: C. Robroeks et al. Metabolomics of Volatile Organic Compounds in Cystic Fibrosis Patients and Controls Pediatric Research 2010, 68, pp. 75–80. DOI: 10.1203/PDR.0b013e3181df4ea0
Disease Area: Cystic Fibrosis, Inflammation, Respiratory Application: Ongoing Monitoring Sample medium: Breath Analysis approach: GC-ToF-MS (Gas Chromatography, Time-of-Flight Mass Spectrometer) Summary:
|
Cystic fibrosis (CF) is a genetic disease that is normally diagnosed at birth via the heel-prick test and affects about one in every 3,000 newborns. People with CF experience a build-up of thick mucus in the lungs and other organs, causing a wide range of symptoms around the body. Around 80% of CF patients eventually die from lung problems.1
This particular study from Robroeks et al. set out to explore the potential of breath biomarkers as tools for ongoing monitoring of CF patients. Diagnosing CF isn’t difficult, but the symptoms caused by CF make it much less easy to detect the emergence of additional pulmonary diseases (such as Pseudomonas colonization) in patients at the earliest and most easily treatable stages. This is particularly true in young children, as existing ongoing monitoring techniques for CF patients are unpleasant and invasive. Late detection of secondary infections can have potentially fatal consequences for CF patients of all ages.
Recently, ongoing monitoring of CF patients has been further complicated by the widespread introduction of the drug TRIKAFTA into treatment plans. Established testing techniques relied on sputum as a source of biomarkers but TRIKAFTA reduces sputum production so as to render sputum sample collection almost impossible in the majority of CF patients. Non-invasive breath monitoring, if successful, has exciting potential to save lives through early detection of new disease.
Airway inflammation is a central process in a number of chronic respiratory diseases, such as cystic fibrosis (CF), asthma, chronic obstructive pulmonary disease and lung cancer, and has the potential to be a key source of breath-based biomarkers for ongoing monitoring.
In the lungs of patients with chronic lung diseases, inflammation can have many different mechanisms and triggers. One common hallmark of inflammation is elevated reactive oxygen species, which break down cell membranes through a process called lipid peroxidation. At least some of the VOCs created by internal metabolic processes have already been shown to be detectable on breath. In their study Robroeks et al. generated models that used breath-based VOCs to differentiate not just CF patients from controls as a first test, but potentially more usefully, also to differentiate CF patients with and without Pseudomonas colonization – a condition which is closely associated with less favorable outcomes for CF patients.
Methods and Analysis
105 children were recruited to participate in the study, of which slightly less than half (48) were CF patients, with 57 controls. Breath samples were initially collected using Tedlar bags before being transferred onto sorption tubes and then capped and stored before analysis via GC-ToF-MS.
Robroeks et al. initially found 1099 molecular features of interest within the study population. Various measures were taken in order to reduce the likelihood of bias in the data, arising from factors like similar medications within the CF group. Repeated measurements of certain participants (after 1 hour and also 1 day) revealed a low level of within-subject variation in VOC patterns, with breathing patterns also offering a low influence.
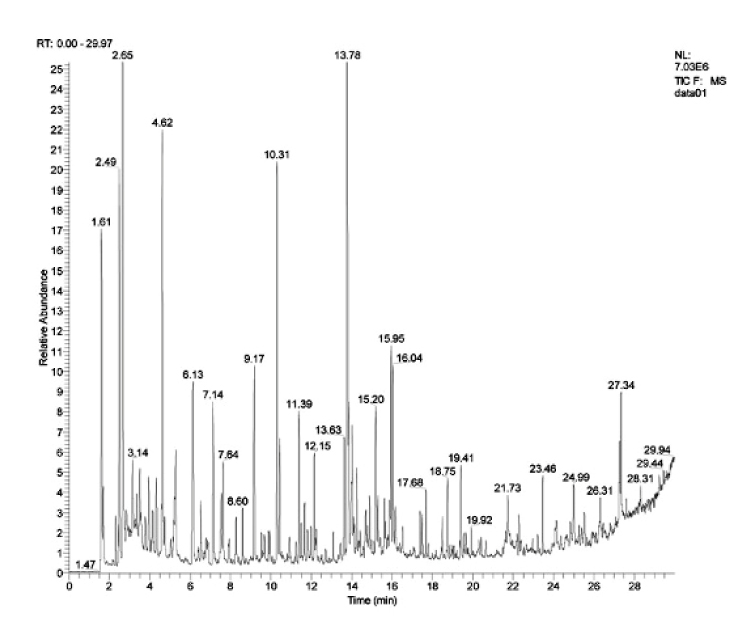
Figure 1: An acquired chromatogram of VOCs.
22 VOCs were found to be particularly useful for discrimination between CF children and controls. A model based on all 22 of these compounds was able to differentiate between these two groups 100% correctly (1.0 AUROC). Of the 10 most significant VOCs, six were prospectively identified as hydrocarbons. Using a further refined model based on these 10, it was possible to discriminate between the two groups with an AUROC of 0.962.
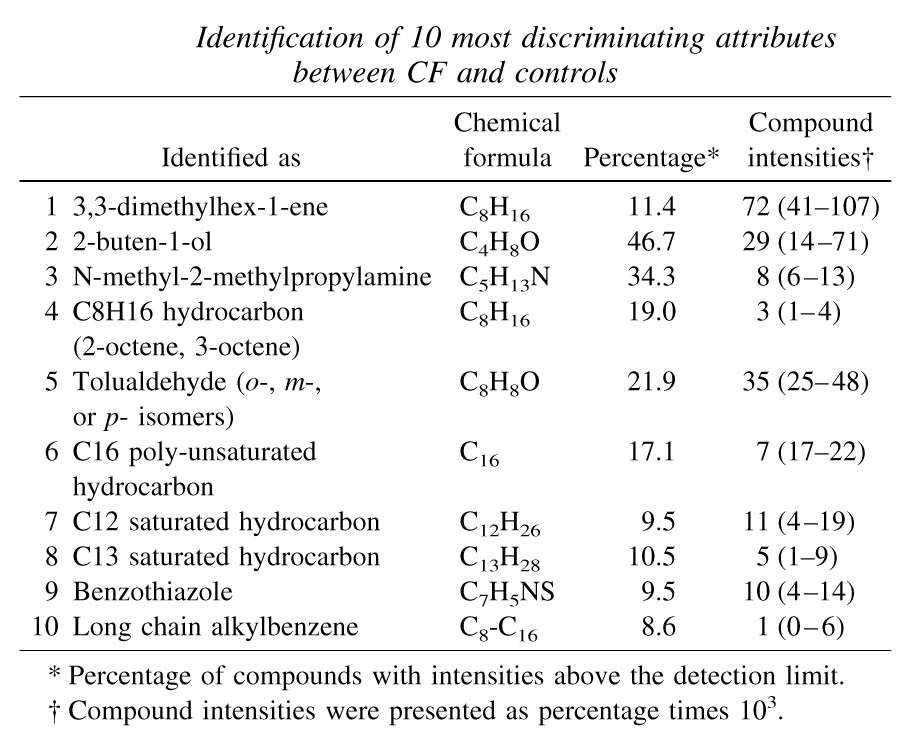 Table 1: Identification of 10 most discriminating attributes between CF and controls |
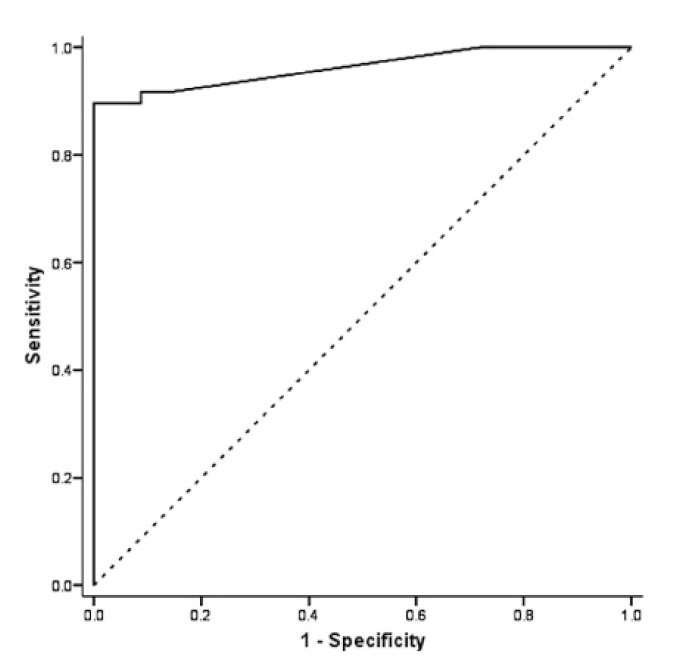 Figure 2: Receiver operating characteristic (ROC) curve of the 10 most prominent attributes discriminating between CF and healthy controls. |
For CF patients, P. aeruginosa infections is linked to increased morbidity and mortality, so the study also wanted to differentiate VOC profiles of CF subjects with and without Pseudomonas colonization. This was found to be possible to do 100% correctly using 14 VOCs in exhaled breath.
Conclusions
One limitation of this study was the lack of any attempt to correct measurements for chemical background, which is necessary to really begin to designate identified compounds as prospective biomarkers. Ambient air contains many VOCs from external sources, and it can be difficult to differentiate these from biorelevant VOCs if they are included during breath sampling. This paper was published in 2010, in the intervening years technologies like CASPER and the ReCIVA Breath Sampler’s Mouthpiece have made keeping background chemicals out of breath samples much easier.
There will be no overnight fixes for CF patients, the authors state that: “Persistent inflammation and repeated cycles of infection are present in CF lungs, resulting in progressive lung damage and pulmonary fibrosis.” However, the results of this study were strong enough for the authors to conclude “VOC profiles may be helpful in the early detection of a CF exacerbation even before symptoms occur and lung function deterioration is present”. The study also underlined the ease and feasibility of using breath collection as a monitoring tool for children, in comparison to some of the more invasive but established sampling methods.
Future research is needed but the authors conclude that “Assessment of VOCs in exhaled breath is a new, promising, noninvasive technique, which, in addition to conventional parameters, can be used to study airway inflammation and oxidative stress in CF patients.”
The Breath Biopsy® Collection Station provides non-invasive and reproducible in vivo sample collection. You can then analyze your CF patients’ samples yourself or send them to the world’s only specialist Breath Biopsy laboratory – offering the most advanced solutions for reliable global breath biomarker analysis.
BREATH BIOPSY FOR RESPIRATORY DISEASES CONTACT US
Additional references
- B. P. O’Sullivan et al. Cystic fibrosis The Lancet 2009, 373, pp1891–1904. DOI: 10.1016/S0140-6736(09)60327-5