Could breath monitoring allow early intervention for COPD exacerbations?
A model based on VOCs has performed comparably to blood biomarkers
| Publication information: Pizzini et al., Analysis of volatile organic compounds in the breath of patients with stable or acute exacerbation of chronic obstructive pulmonary disease, J. Breath Res. (2018) 12. 036002. DOI: 10.1088/1752-7163/aaa4c5
Disease Area: Respiratory, Chronic obstructive pulmonary disease (COPD) Application: Personalized medicine Sample medium: Breath Analysis approach: TD-GC-ToF-MS Summary:
|
The WHO estimates that by 2030 chronic obstructive pulmonary disease could be the third greatest cause of deaths worldwide. Patients with COPD experience some day-to-day change in respiratory symptoms. A larger than normal worsening of symptoms is known as an acute exacerbation of COPD (AECOPD) and is especially associated with worse clinical outcomes from reduced quality of life to significantly increased likelihood of death.
In COPD patients, AECOPD is often caused by infectious or bacterial pathogens and is frequently treated with a change in medication. Diagnosis of AECOPD is currently problematic and mainly determined by clinical criteria, such as increased dyspnea, increased sputum volume, and increased coughing and wheezing. More invasive methods of investigating bronchial inflammation are not as sensitive as might be expected, complicated by the inflammatory background of COPD patients, and not practical for routine use with this patient group.
Currently no AECOPD biomarkers are in use clinically. In the absence of an established biomarker, C-reactive protein (CRP), a non-specific measure of inflammation in the body, has been suggested as a surrogate parameter. Uncertainty in diagnosis leads to overuse of precautionary antibiotics, increasing patient’s resistance to conventional drugs and the prevalence of related side effects.
If a non-invasive biomarker could be identified on exhaled breath, capable of differentiating between COPD and AECOPD, it would make diagnosis significantly easier and more accurate, while improving patients’ quality of life and reducing unnecessary healthcare spending. A miniaturized sensor device could even allow precision monitoring of COPD patients in the home, detecting exacerbations in time for earlier intervention and improved outcomes.
In this pilot study Pizzini et al. investigated the diagnostic potential of breath VOCs to detect AECOPD in contrast to clinically stable COPD patients.
Methods
30 patients (14 AECOPD and 16 COPD) and 24 healthy controls were recruited for this study. Stable COPD patients were recruited during routine check-ups where no acute exacerbation or infection was detected. AECOPD patients were diagnosed using GOLD AECOPD criteria ‘based on clinical and anamnestic findings, requiring hospitalization or change in medication’. For the AECOPD group breath collection was completed within 48 hours of hospitalization and blood samples for all groups were collected within the same 24 hour window as breath samples.
Breath samples were collected from only the end-tidal exhalation phase, when it is believed ambient contamination will be reduced. Duplicate samples were collected of the air at the sampling location, for comparison. Breath samples were pre-concentrated on sorbent tubes before being analyzed by thermal desorption gas chromatography time-of-flight mass spectrometry (TD-GC-ToF-MS) on the same day. VOC identities were assigned with reference to the NIST 2008 mass spectral library and then confirmed with pure standards.
Results
In the analysis, 105 VOCs were initially targeted. Only VOCs appearing in at least 30% of samples selected for further analysis. After comparing relative abundance of VOCs between patient groups, Pizzini et al. then further narrowed their focus to 22 which appeared to demonstrate some potential for differentiation, on the basis of calculated p-values and the corresponding q-values. They then excluded 6 VOCs on the basis of their close connection with smoking in the literature (this does not preclude any of the remaining compounds from having a bias associated with smoking) and a further 4 VOCs which were found to be less common on exhaled breath than ambient air (having calculated an alveolar gradient).
Of the remaining 12 VOCs, n-butane, 2-pentanone, cyclohexanone and 4-heptanone were found to be potentially useful for differentiating AECOPD, with the three ketones at raised levels in the breath of AECOPD patients and n-butane present at reduced levels. Higher levels of n-Heptane and methyl propyl sulfide appeared to indicate stable COPD. Dimethyl disulfide and 6-methyl-5-hepten-2-one demonstrated some ability to differentiated health volunteers from both COPD or AECOPD. The remaining 4 VOCs were discriminative for only one COPD group from controls.
Using these 12 VOCs a classification model was developed, which had an area under the ROC curve of 0.97, with a sensitivity of 0.78 and specificity of 0.91. A secondary model that used just 4 VOCs to differentiated AECOPD and COPD had an area under the ROC curve of 0.92, with slightly reduced sensitivity but improved specificity.
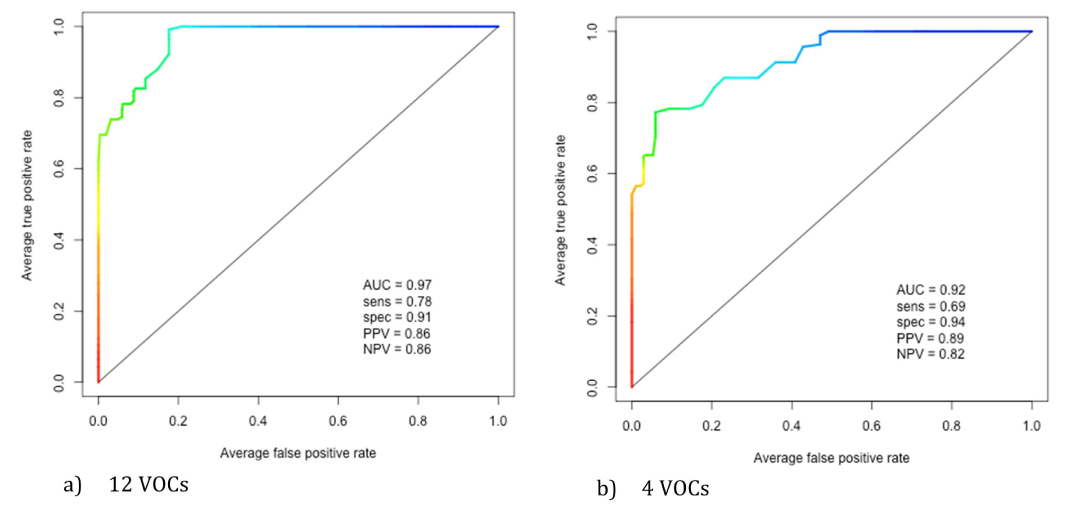
Figure 1. ROC curve illustrating sensitivity and 1—specificity for binary classification of AECOPD and COPD patients. (a) represents the ROC curve for the 12 preselected VOCs. (b) represents the ROC curve for four AECOPD specific VOCs.
The metabolic sources of the three ketones (cyclohexanone, 2-pentanone and 4-heptanone) which were found to differentiate AECOPD from COPD are currently unknown. Further translational research would need to be done to establish their origins before a clinical test could be developed, from in vitro cultivated bacteria and animal models to human breath research. However, two of those VOCs (2-pentanone and 4-heptanone) have previously been found to be released by pathogenic bacteria causing various type of pneumonia, often found in AECOPD patients, with concentrations linked to severity of infection – so these early results are promising.
The breath VOC classification model (with it’s AUC of 0.97) performed better than equivalent results that have been calculated using conventional non-specific blood serum markers, such as CRP. The potential speed of results that a breath test based on a sensor could produce, coupled with breath’s inherent non-invasiveness as a sample would make it the preferable solution for ongoing monitoring with a COPD patient group. Developing a simpler test would allow for reduced complexity in the analytical pipeline which is why Pizzini et al. developed the second model based on only 4 VOCs.
Further work and larger scale studies are needed to validate these results because this small pilot study is potential confounded by several factors including age (the control cohort is substantially younger than the disease groups), smoking status and pack years. There is also no mention in this study of coincident diseases in the cohort, even though the COPD population is often also affected by diabetes and other cardiovascular and metabolic diseases.
Pizzini et al. believe that ‘the future of medical diagnosis of lung diseases, including AECOPD, [lies] in the non-invasive and personalized monitoring of biomarkers in exhaled air’ and the results of this pilot study seem to support that idea.
At Owlstone Medical we agree with Pizzini et al. that identifying and selecting relevant breath VOCs is ‘the first and essential step towards the identification of useful biomarkers’ and we’ve developed a comprehensive and robust global breath VOC analysis.
If you’d like to work with us to investigate biomarkers for COPD or another disease, please get in touch. We can also support in vitro experiments, highlighted by Pizzini et al. as an important next step to validate their findings.
