Analytical and Sensor-led Approaches to Breath Research
Published on: 8 Sep 2020
The aim of any clinical study in breath biomarker research is to detect changes in exhaled breath that can be associated with biological factors such as disease progression or pharmacological interactions. Breath is a complex medium containing many different substances with the potential to be used as biomarkers, some of which are only present in breath at the parts per trillion level.
In many fields, breath research is in its early stages. That means that identifying breath biomarkers, that are demonstrably significant in relevant metabolic processes is of vital importance. Two predominating approaches have emerged as tools for breath research in the clinical setting.
What approaches do you use for breath sample analysis? Visit our community to let us know.

The first approach, performs a qualitative assessment of the exhaled breath composition, usually by an individual chemical sensor or arrays of specific or cross-reactive chemical sensors. The signals obtained can be translated via mathematical algorithms into discriminative breath-prints or patterns associated with a specific group of patients/diseases. Inspired by the mammalian sense of smell, such systems have sometimes been referred to as Electronic Noses (eNoses).
The second, performs quantitative analytical characterization, by means of in-depth techniques such as gas chromatography-mass spectrometry (GC-MS). Through these tools, the (volatile organic compounds) VOCs in exhaled breath are separated, identified, and quantified. This approach reveals individual VOCs, which can be investigated, either individually or in combination, as prospective biomarkers.
The Strengths and Limitations of Sensor-led Analysis
Sensor-based qualitative systems are portable, robust, easy to use, and represent an economical option for breath analysis. They can provide near-immediate results avoiding the delays involved in having to ship collected breath samples somewhere else for analysis and interpretation – making them preferable for point-of-care use.
When analyzed by a sensor-based system, each sample will produce a unique breath print. Breath prints from different groups can then be compared to identify characteristic features for each group. However, sensor-based systems do not provide an insight into what a breath-print means, or which analytes are responsible for the distinctive signals. As such it is impossible to determine the disease relevance of the identified features and they cannot be reliably distinguished from other factors that may vary in the population.
Further, sensor-based systems will only detect what they’re designed to detect, so a lack of biomarker knowledge in advance will make it harder to adapt/optimize the device for a specific application. This could result in relevant factors being completely missed by the device. When sensor technologies are used in a discovery study it is therefore necessarily being assumed:
[…] that the biomarker panel detected with that specific technology in an initial discovery phase directly translates, without alteration, to the intended use population and all future devices of the same type. Understandably, to-date no one has been able to prove this without understanding the underlying compounds […].
Beauchamp et al. (2020) [1]
When testing sensor-based technologies as diagnostic tools, it’s therefore vital that those studies are performed within the intended use population, rather than subsequently attempting to translate a study’s findings to other groups. If this doesn’t happen, it will likely be impossible to later validate any derived response profiles.
If you already know what the relevant biomarkers for a given disease are you could develop tailor-made sensors, with high sensitivity and specificity for those biomarkers, in order to examine the differences between populations. Ultimately, to get a clinical diagnostic test approved it will always be necessary to demonstrate a relationship between your test and the disease, which is hard to do without biological knowledge of your identified diagnostic features.
Conducting Quantitative Biomarker Discovery Studies
 |
There are many different tools that can be used for quantitative analysis of exhaled breath, but the most effective is Gas Chromatography Mass Spectrometers (GC-MS). Compared to employing sensor-based systems this kind of analysis offers a much more detailed and richer set of results but is also more costly and time-consuming. Such approaches require highly specialist laboratories and experienced analytical chemists – which for many studies can mean sending samples off-site for analysis. |
Unlike sensor-based systems, quantitative tools such as GC-MS can accurately identify individual analytes present in breath samples. At this relatively early stage in the development of the field, we believe the ability to identify and investigate the role of specific biomarkers is a key advantage in developing effective breath tests for clinical applications.
A key benefit of the quantitative approach is that, once biomarkers have been identified and thoroughly validated, a wider range of options are available for their subsequent development. Well-characterized biomarkers can be investigated within differing patient populations, irrespective of whether the same technology is used for analysis. It is this level of versatility that makes quantitative discovery methods extremely advantageous when creating a clinical test that could be approved by regulatory bodies.
Biological knowledge also allows you to optimize your whole collection and detection process to your identified biomarkers. One example of this is using a well-characterized biomarker originating outside the body as an Exogenous Volatile Organic Compound Probe (EVOC® Probe). You could also change the sorbent tubes used for collection, the GC column, and even the MS method; to better detect those biomarker compounds that you’re interested in.
Significantly, once biomarkers have been isolated quantitatively you can then also progress to designing qualitative sensors capable of targeting the specific biomarkers you’ve identified. This combined approach makes it possible to take advantage of both the quantitative and sensor-based tools for breath analysis. Underpinned by sufficient biological knowledge, the immediate yes/no response provided by a tailor-made sensor-based system provides rapid results valued for clinical use supported by thorough biological evidence.
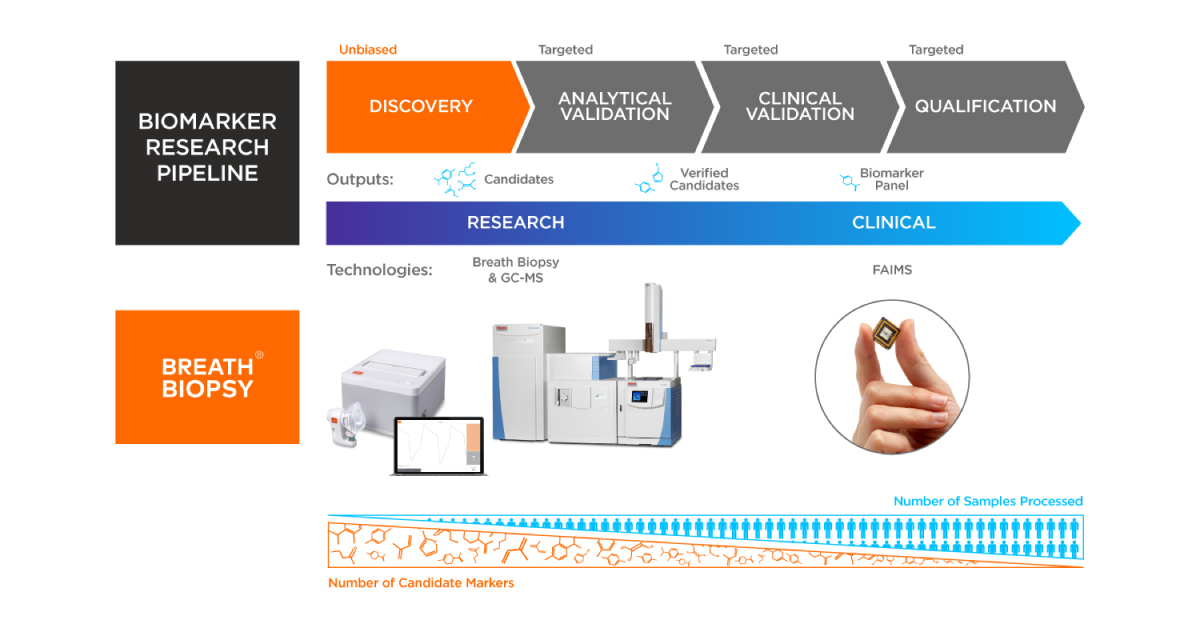
Owlstone Medical’s Approach to Breath Research
We’ve designed and built our Breath Biopsy® Platform to accommodate the robust discovery powers of quantitative techniques while also reflecting the long-term need to deliver convenient clinical solutions.
Reliable and consistent sample collection is key to ensuring high-quality breath analysis and biomarker identification. We’ve created the ReCIVA® Breath Sampler to allow standardization in sample collection and CASPER™ Portable Air Supply to reduce the level of background VOCs that might interfere with genuine biomarker signals in a collected sample.
The Breath Biopsy Platforms then provide robust, high-resolution analysis thanks to the fully equipped Breath Biopsy Laboratory with GC-MS Orbitrap capabilities. As the field progresses, our expertise in FAIMS technology, a qualitative sensor-led system, means we are also able to support development towards point-of-care solutions.
We’re Here to Help
Our expert team of clinical and technical specialists can provide comprehensive support for all your studies and offer a complete set of services for the analysis of non-invasive biomarkers in breath.
Reference
- Beauchamp et al., Breathborne Biomarkers and the Human Volatilome, 2nd edition, Elsevier, 2020, ISBN: 9780128223970