Hydrogen Sulphide and its Role in Gastrointestinal Disease
Published on: 11 Jul 2022
If you are a patient looking for further information on Hydrogen Sulphide – read the blog, ‘Hydrogen sulfide and what it can tell you about your gut health,’ via our OMED Health website.
Hydrogen sulphide (H2S) is a gas produced exogenously and endogenously in humans, including by sulphate reducing bacteria (SRB) in the gut. H2S is a gut health regulator that influences motility, ischemia, and reperfusion, and can promote or inhibit inflammation depending on concentration1, 2.
Excessive levels of SRB in the intestinal lumen may cause H2S small intestinal bacterial overgrowth (SIBO)3 or colonic dysbiosis. Alterations in H2S levels have been linked to gastrointestinal disorders such as ulcerative colitis (UC), Crohn’s disease (CD), and irritable bowel syndrome (IBS)3. Patients with diarrhea tend to show higher levels of exhaled H2S4. In the colon, approximately 66% of SRB are Desulfovibrio and 16% are Desulfobulbus5, although there is evidence that SRB composition varies in patients with gastrointestinal symptoms6, 7.
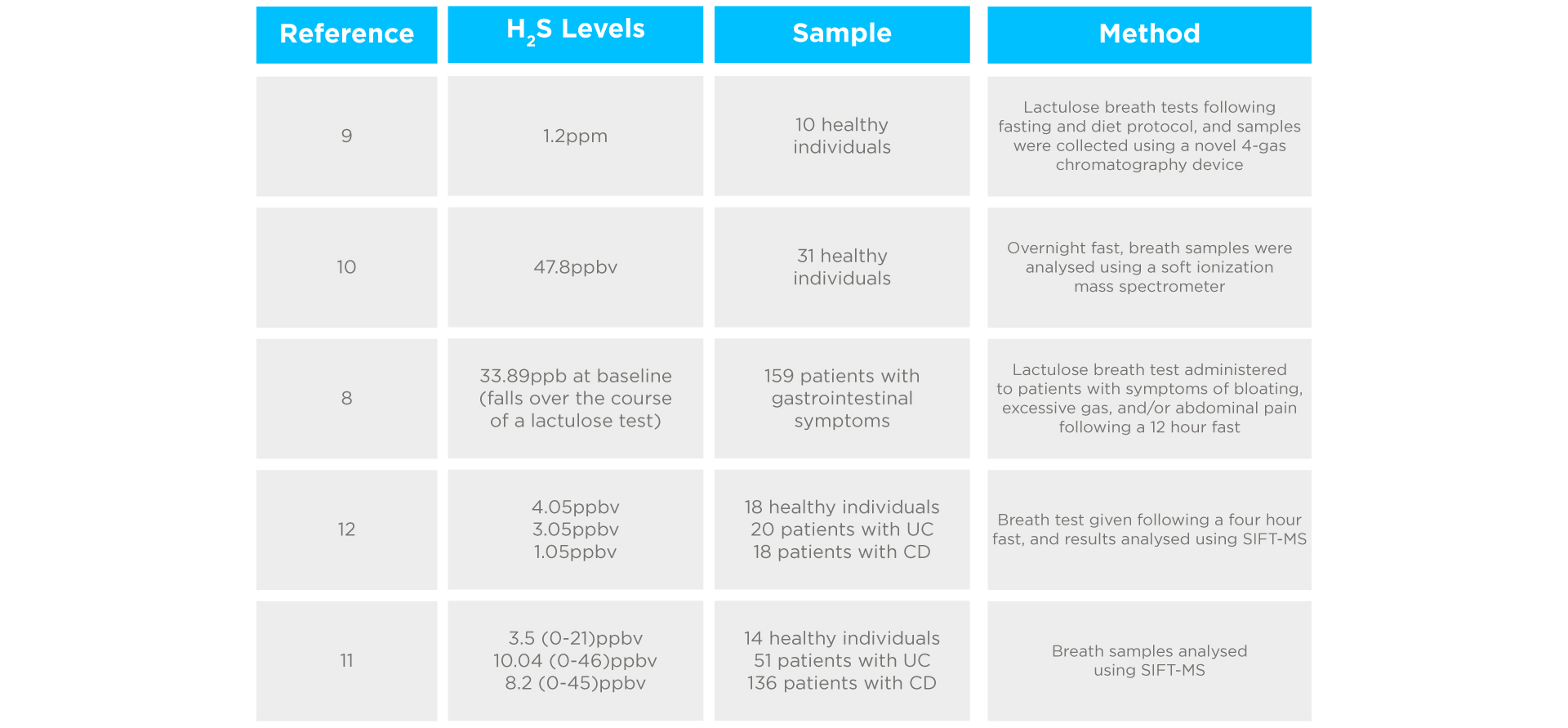
H2S levels can be detected in exhaled breath, as it travels from the gut to the lungs via the bloodstream8. In healthy individuals, H2S is observed at a rate below 1.2ppm in the breath9. Breath H2S levels ≥1.2ppm are clinically significant and correlate with diarrhoea, urgency, and abdominal pain. Lower estimates for H2S in healthy individuals have also been reported, including 47.8ppbv10, 3.5ppbv11, and 4.05ppbv12. In contrast, patients referred for breath testing due to gastrointestinal symptoms showed mean H2S levels of 33.89ppb at baseline, which fell over the course of a lactulose breath test8. A study of 187 patients with IBD (both CD and UC) showed higher levels and a wider spread of H2S in the breath compared to healthy controls11.
Interestingly, no link was found between disease severity and H2S concentration. A rise of H2S to ≥25.0ppbv, or H2S levels at 90 minutes ≥62.5ppbv following a lactulose breath test has been considered positive for H2S SIBO13. However, findings have not been uniform, with some contradictory evidence suggesting patients with UC and CD show significantly lower H2S breath concentrations than healthy subjects (3.05, 1.05, and 4.05ppbv respectively)12. Please see a summary below in table 1. Higher H2S concentrations are observed in mouth-exhaled breath than nose-exhaled breath due to bacterial H2S production in the oral cavity, so testing protocols can have a substantial impact on outcomes14.
Studies investigating H2S levels in exhaled breath
Hydrogen is produced in the gut by microbes such as Bacteroidetes, with SRB and methanogens (which produce methane) competing for this hydrogen8. It is estimated that approximately 40% of people use methanogenesis and 55% use sulphate reduction as their primary hydrogen consumptive pathway16. The more hydrogen that is converted to methane and H2S, the less is detected in exhaled breath. However, H2S is not routinely measured in breath tests.
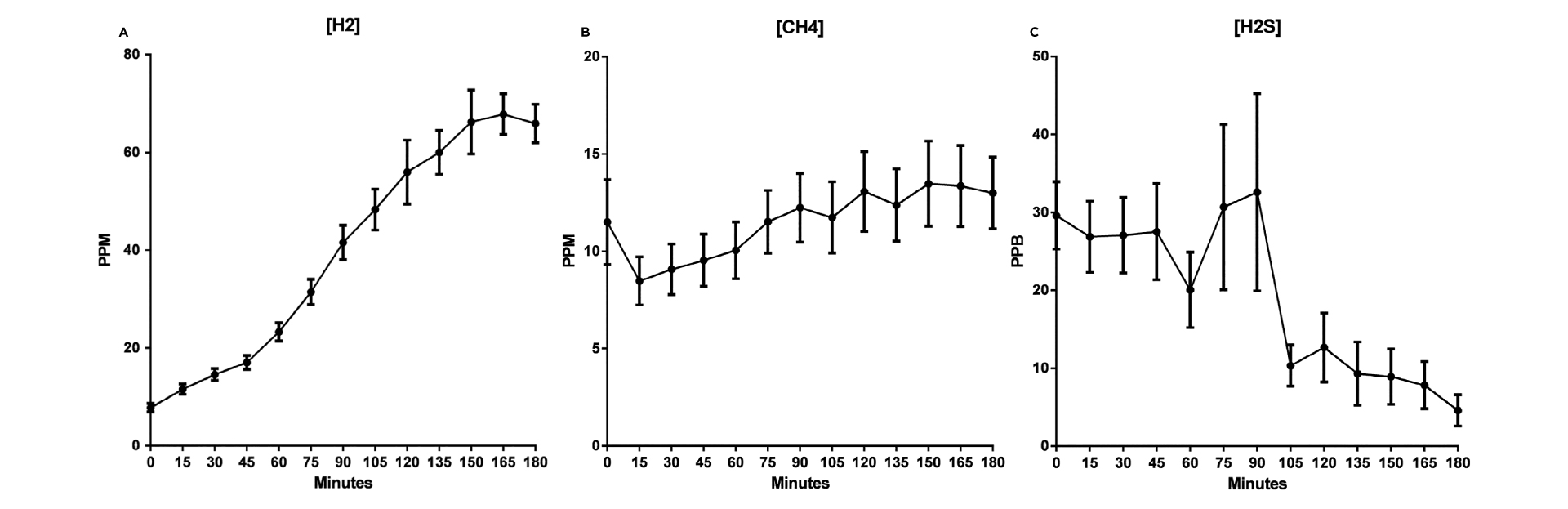
A study by Birg et al. (2019) that used lactulose breath tests to assess levels of hydrogen, methane, and H2S in patients with gastrointestinal symptoms found that unlike methane and hydrogen, H2S showed a decreasing concentration over the course of the breath test(see figure 1). Additionally, the authors suggested that patients may show a ‘flatline’ hydrogen profile if hydrogen production is within the saturation threshold for hydrogen consumers (methanogens and SRB). Therefore, a lack of exhaled hydrogen and a negative result does not necessarily mean that hydrogen production was within normal limits, and relying on methane levels as the only indicator of hydrogen consumption may lead to misleading breath test results8.
Concentrations of methane, H2S, and hydrogen following a lactulose challenge in patients referred for breath testing8
There is evidence that SRB levels contribute to bowel inflammation and developing UC via H2S production17. Studies show patients with IBD have greater concentrations of SRB than healthy individuals in the gut which is consistent with raised H2S levels in breath tests18, 19, 20. Patients with IBD have higher levels of SRB in their feces (68%) compared to controls (24%)18, and previous research has produced more extreme estimates, such as the finding that 96% of patients with UC but only 50% of healthy subjects had significant SRB populations19. The mucosal bacteria population of IBD patients also houses a higher proportion of SRB than in healthy controls20.
The correlation between H2S and IBD has been suggested to be due to the damaging effects of H2S on gut mucosa via butyrate oxidation inhibition21. Fiber fermentation in the gut produces butyrate, a short-chain fatty acid (SCFA). Oxidation of SCFAs, primarily butyrate, provides approximately 60-70% of colonocyte energy22. Impairment of butyrate oxidation could lead to cell death and chronic inflammation6. This is consistent with the ‘energy deficiency’ hypothesis that argues UC occurs due to butyrate oxidation inhibition23 and is supported by evidence that patients with active UC have reduced butyrate oxidation compared to healthy controls24. In Hond’s (1998) study, all patients with inactive UC coupled with reduced oxidation relapsed within a few weeks while patients with inactive UC and normal oxidation did not relapse for at least three months.
There is less research investigating the role of H2S in IBS than IBD, however, diarrhoea-predominant IBS patients have been found to show altered H2S levels in the breath3. Rat models have also shown cystathionine-β-synthetase (CBS), an H2S-producing enzyme, is upregulated in IBS-like chronic visceral hyperalgesia25. CBS has therefore been suggested as a potential target for treating visceral pain in IBS.
Further research into treatments for excessive H2S production is needed. It is currently unclear if treatments for other forms of SIBO, such as Rifaximin, are effective against H2S SIBO due to a lack of data. A low sulphide diet, excluding foods such as sausage, beer, wine, bread, dried fruit, and soya flour, has been suggested to reduce H2S production26. A small study of six methane-producing participants found that dietary sulphate may enable the growth of SRB, which in turn inhibit methanogenic bacteria27. Similarly, a pilot study of eight UC patients who eliminated dietary sulphur-containing amino acids and reduced red meat intake showed no relapses or attacks over the following year, despite an expected relapse rate of 22.6%28.
In a recent survey of >400 patients referred for HMBT, 17% reported weekly increased flatulence and diarrhea. In this sub-group of patients, approximately 30% had a normal breath test in terms of being negative for SIBO and IMO. In addition, 6% of patients had a ‘flatline’ breath test, meaning no significant detectable gas was measurable apart from CO2, confirming the validity of the samples. Whilst there may be other causes for these symptoms / findings such as bile acid mal-absorption, carbohydrate mal-absorption or slow intestinal transit, the addition of H2S detection to our diagnostic portfolio will help us to reduce the number of potential false negative test results.
In summary, H2S may well be an important breath biomarker of intestinal health and dysbiosis.
Hydrogen sulphide healthy subject study
We recently completed an assessment of H2S levels in healthy subjects. Participants were eligible to take part if they were aged 18-60 years, were not pregnant or breastfeeding, had no significant medical problems, did not take regular prescription medication, did not regularly use laxatives or promotility agents, and agreed to adhere to all preparations required for the breath test.
Before the breath test, all participants were asked to adhere to a low fermentable diet in the 24 hours prior to the breath test and a 12-hour fast. Participants also must not have had any antibiotics in the 4 weeks prior to study enrolment, stopped any probiotics, laxatives, stool softeners, or promotility agents, and not had a test that requires bowel cleansing prior (e.g. colonoscopy) for 1 week before the test. Participants were instructed to avoid eating, drinking, smoking, exercising, and sleeping during the test.
Participants provided normal expiratory baseline breath samples before ingesting 10g lactulose dissolved in 200 ml of water. Further breath tests were collected at 15-minute intervals until 180 minutes had passed, for hydrogen and methane analysis, and at 45, 90, and 180 minutes post lactulose ingestion for hydrogen sulphide analysis.
Hydrogen and methane breath samples were collected using the Functional Gut collection kit by exhaling into a breath test tube for 3-5 seconds, and hydrogen sulphide samples were collected by exhaling into 500ml polyvinylidene fluoride bags. Hydrogen and methane samples were analyzed using gas chromatography and H2S via Syft-MS.
Results
Graphs for mean hydrogen, methane, and hydrogen sulphide are shown below. Whilst hydrogen and methane levels increased over the course of the study as the lactulose was fermented, hydrogen sulphide production decreased over the time course of the breath test, which is in agreement with findings from Birg et al 2019 and may be a feature of a healthy microbiome30. Hydrogen sulphide levels were consistently below 50ppb which allows us to establish the normal range that can be used in future clinical studies.
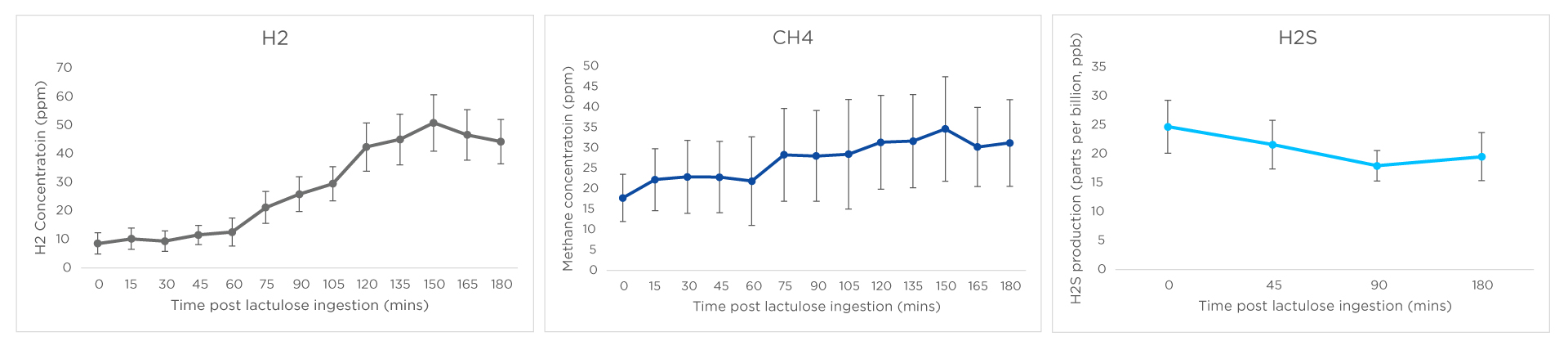
Figure 2: Mean hydrogen (H2), methane (CH4) and hydrogen sulphide (H2S) production in parts per million (ppm) for hydrogen and methane, and parts per billion (ppb) for hydrogen sulphide over a 3hr lactulose breath test.
References:
1. Blachier, F., Beaumont, M. and Kim, E., 2019. Cysteine-derived hydrogen sulfide and gut health. Current Opinion in Clinical Nutrition & Metabolic Care, 22(1), pp.68-75.
2. Singh, S. and Lin, H., 2015. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms, 3(4), pp.866-889.
3. Banik, G., De, A., Som, S., Jana, S., Daschakraborty, S., Chaudhuri, S. and Pradhan, M., 2016. Hydrogen sulphide in exhaled breath: a potential biomarker for small intestinal bacterial overgrowth in IBS. Journal of Breath Research, 10(2), p.026010.
4. Pimentel, M., Hosseini, A., Chang, C., Mathur, R., Rashid, M., & Sedighi, R. et al. (2021). Fr248 Exhaled Hydrogen Sulfide Is Increased In Patients With Diarrhea: Results Of A Novel Collection And Breath Testing Device. Gastroenterology, 160(6), S-278. doi: 10.1016/s0016-5085(21)01391-3
5. Linden, D., 2014. Hydrogen Sulfide Signaling in the Gastrointestinal Tract. Antioxidants & Redox Signaling, 20(5), pp.818-830.
6. Loubinoux, J., Bronowicki, J., Pereira, I., Mougenel, J. and Faou, A., 2002. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiology Ecology, 40(2), pp.107-112.
7. Gibson, G., Cummings, J. and Macfarlane, G., 1991. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiology Letters, 86(2), pp.103-112.
8. Birg, A., Hu, S. and Lin, H., 2019. Reevaluating our understanding of lactulose breath tests by incorporating hydrogen sulfide measurements. JGH Open, 3(3), pp.228-233.
9. Fowler, H., Pichetshote, N., Hosseini, A., Takakura, W., Sedighi, R., Chang, C., Pimentel, M., Mathur, R. and Rezaie, A., 2020. Su1211 The First Study To Determine The Normal Range Of Exhaled Hydrogen Sulfide (H2s) Using A Novel 4-Gas Breath Test Device In Healthy Subjects. Gastroenterology, 158(6), p.S-545.
10. Morselli-Labate, A., Fantini, L. and Pezzilli, R., 2007. Hydrogen Sulfide, Nitric Oxide and a Molecular Mass 66 u Substance in the Exhaled Breath of Chronic Pancreatitis Patients. Pancreatology, 7(5-6), pp.497-504.
11. Dryahina, K., Smith, D., Bortlík, M., Machková, N., Lukáš, M. and Španěl, P., 2018. Pentane and other volatile organic compounds, including carboxylic acids, in the exhaled breath of patients with Crohn’s disease and ulcerative colitis. Journal of Breath Research, 12(1), p.016002.
12. Hicks, L., Huang, J., Kumar, S., Powles, S., Orchard, T., Hanna, G. and Williams, H., 2015. Analysis of Exhaled Breath Volatile Organic Compounds in Inflammatory Bowel Disease: A Pilot Study. Journal of Crohn’s and Colitis, 9(9), pp.731-737.
13. Guo, H. Z., Dong, W. X., Zhang, X., Zhu, S. W., Liu, Z. J., & Duan, L. P. (2021). Zhonghua nei ke za zhi, 60(4), 356–361. https://doi.org/10.3760/cma.j.cn112138-20200731-00725
14. Dryahina, K., Smith, D., Bortlík, M., Machková, N., Lukáš, M. and Španěl, P., 2017. Pentane and other volatile organic compounds, including carboxylic acids, in the exhaled breath of patients with Crohn’s disease and ulcerative colitis. Journal of Breath Research, 12(1), p.016002.
15. Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. Journal of Neurogastroenterology and Motility. 2014; 20: 31–40.
16. Kushkevych, I., Dordević, D. and Kollár, P., 2019. Analysis of physiological parameters of Desulfovibrio strains from individuals with colitis. Open Life Sciences, 13(1), pp.481-488.
17. Fite, A., 2004. Identification and quantitation of mucosal and faecal desulfovibrios using real time polymerase chain reaction. Gut, 53(4), pp.523-529.
18. Florin, T.HJ, Gibson, G.R., Neale, G. and Cummings, LH. (1990) A role for sulfate reducing bacteria in ulcerative colitis? Gastroenterology 98, AIT0. In Gibson, G., Cummings, J. and Macfarlane, G., 1991. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiology Letters, 86(2), pp.103-112.
19. Kleessen, B., Kroesen, A., Buhr, H. and Blaut, M., 2002. Mucosal and Invading Bacteria in Patients with Inflammatory Bowel Disease Compared with Controls. Scandinavian Journal of Gastroenterology, 37(9), pp.1034-1041.
20. Dordević, D., Jančíková, S., Vítězová, M. and Kushkevych, I., 2021. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. Journal of Advanced Research, 27, pp.55-69.
21. Brahe, L., Astrup, A. and Larsen, L., 2013. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases?. Obesity Reviews, 14(12), pp.950-959.
22. Roediger, W., 1980. The Colonic Epithelium In Ulcerative Colitis: An Energy-Deficiency Disease?. The Lancet, 316(8197), pp.712-715.
23. Hond, E., Hiele, M., Evenepoel, P., Peeters, M., Ghoos, Y. and Rutgeerts, P., 1998. In vivo butyrate metabolism and colonic permeability in extensive ulcerative colitis. Gastroenterology, 115(3), pp.584-590.
24. Xu, G., Winston, J., Shenoy, M., Zhou, S., Chen, J. and Pasricha, P., 2009. The Endogenous Hydrogen Sulfide Producing Enzyme Cystathionine-β Synthase Contributes to Visceral Hypersensitivity in a Rat Model of Irritable Bowel Syndrome. Molecular Pain, 5, pp.1744-8069-5-44.
25. Kushkevych I, Dordević D, Kollar P, Vítězová M, Drago L. Hydrogen Sulfide as a Toxic Product in the Small-Large Intestine Axis and its Role in IBD Development. Journal of clinical medicine. 2019;8(7):1054.
26. Christl, S., Gibson, G., & Cummings, J. (1992). Role of dietary sulphate in the regulation of methanogenesis in the human large intestine. Gut, 33(9), 1234-1238. doi: 10.1136/gut.33.9.1234
27. Roediger W. Decreased sulphur amino acid intake in ulcerative colitis. The Lancet 1998;351:1555.
28. Eisenmann A., Amann A., Said M., Datta B., Ledochowski M. (2008) Implementation and interpretation of hydrogen breath tests. J. Breath Res. 2 046002 (9pp).
29. Rezaie A., Buresi M., Lembo A., Lin H., McCallum R., Rao S., Schmulson M., Valdovinos M., Zakko S., Pimentel M. (2017) Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. A m J Gastroenterol 112:775–784. doi: 10.1038/ajg.2017.46
30. Birg A., Hu S., C Lin H. (2019) Reevaluating our understanding of lactulose breath tests by incorporating hydrogen sulfide measurements. JGH Open: An open access journal of gastroenterology and hepatology 3 228–233. doi:10.1002/jgh3.12145