How are volatile compounds detected and measured in exhaled breath?
Published on: 7 Jul 2023
In the fourth installment of our introductory blog series, we will cover:
- How are breath samples collected?
- How are breath samples analyzed?
We have previously discussed why breath analysis can provide many unique advantages as a biological sample, what compounds can be found in the breath, and what processes they originate from, but how does breath analysis actually work? In this blog post, we will introduce how compounds in breath can be collected, analyzed, and measured for their use as biomarkers for clinical use. For an overview of how we conduct our breath analysis workflow, please see our video below.
How are breath samples collected?
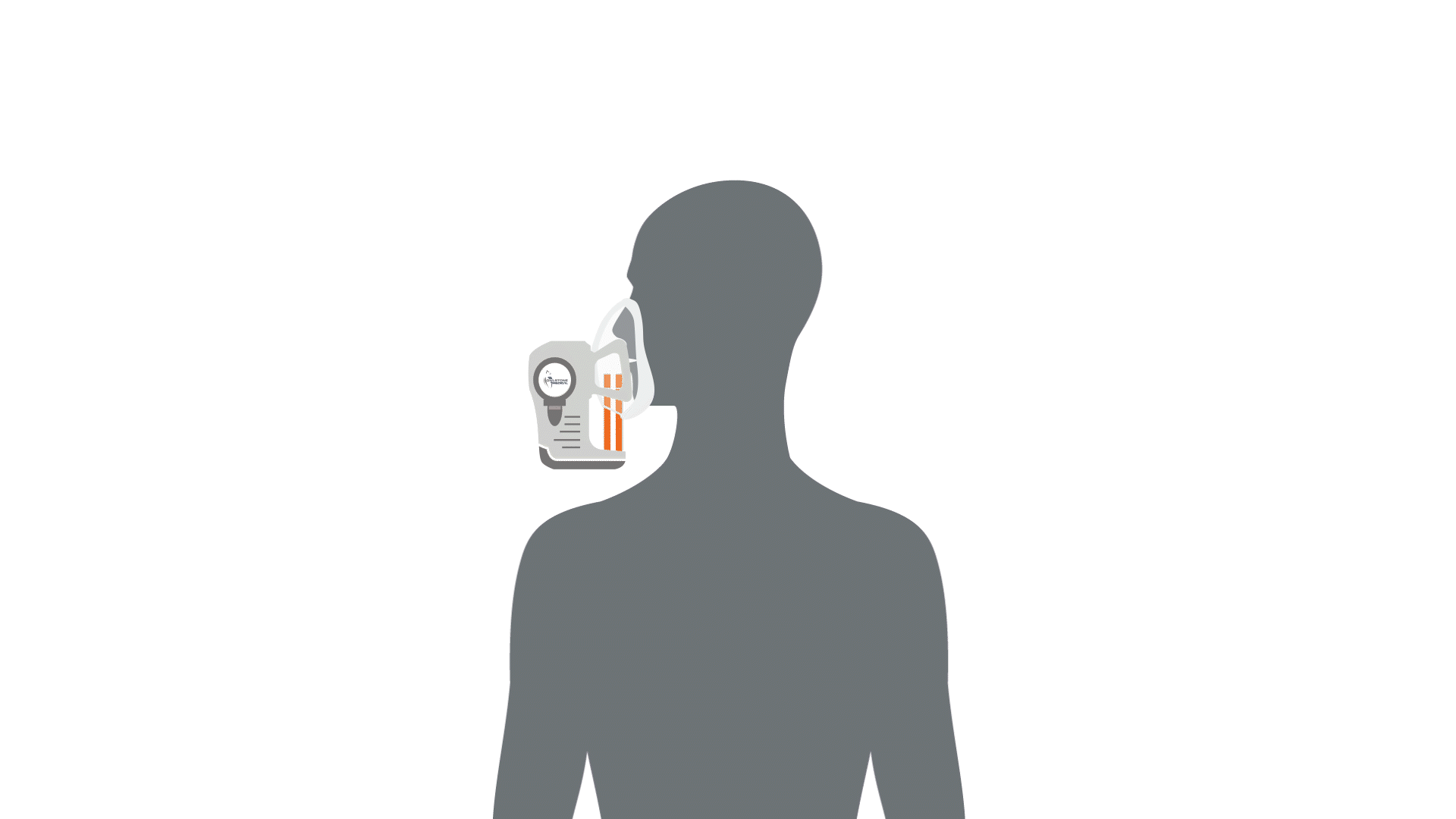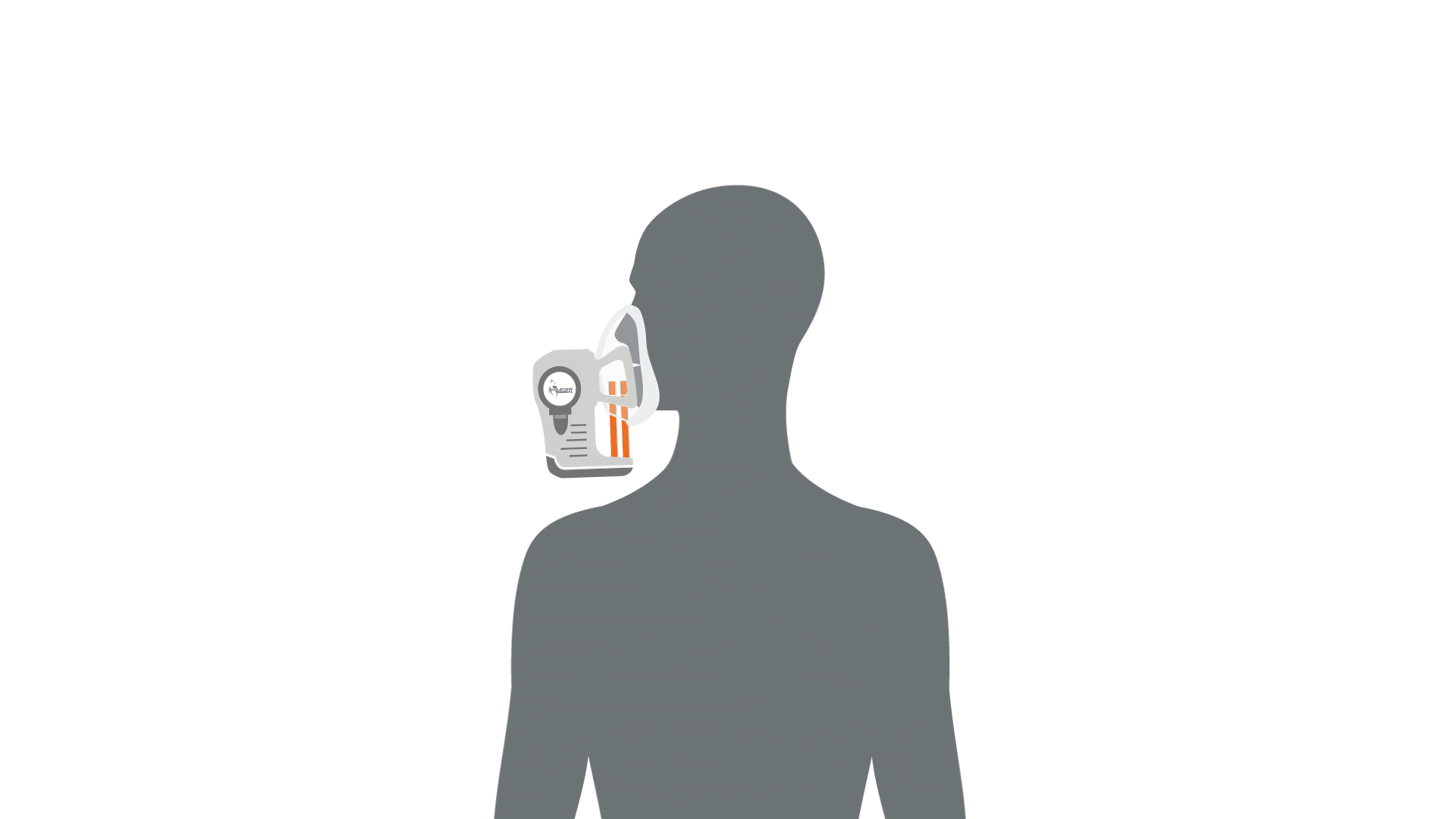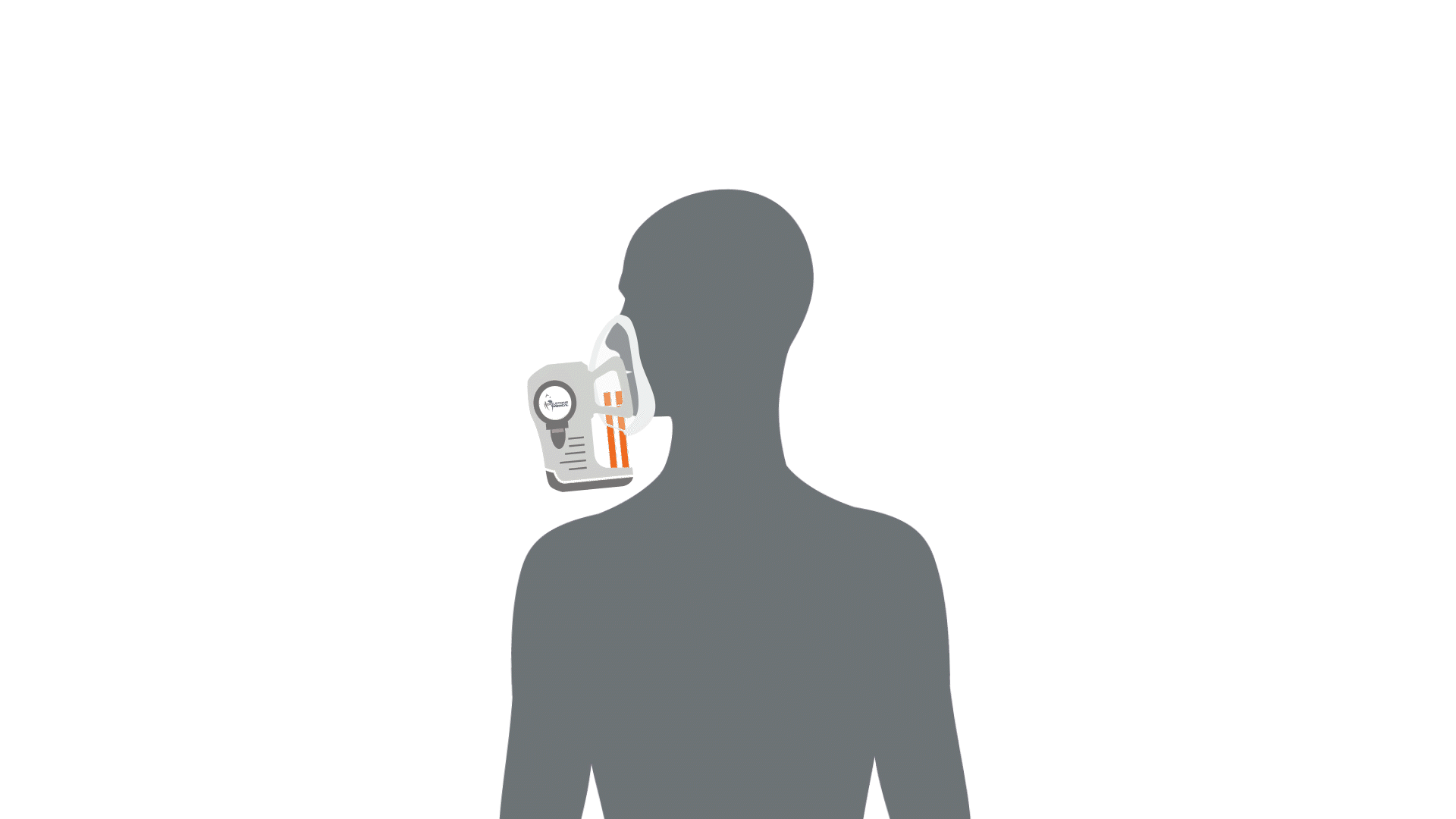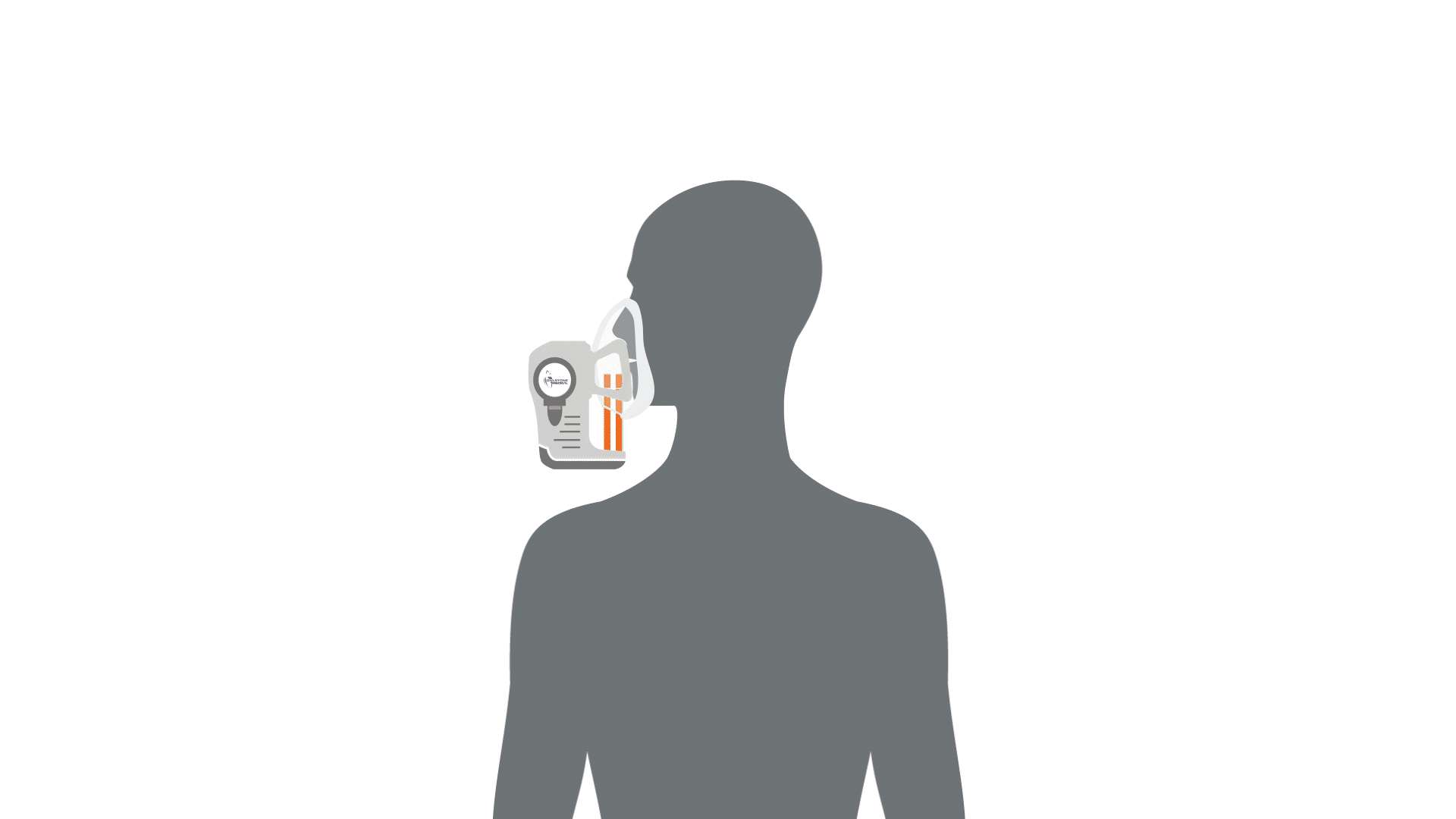
The first stage of breath analysis is to collect a sample of breath. There are a few different mechanisms that can be utilized to collect breath such as exhalation into Tedlar bags. However, studies have shown that these bags can be a source of contaminating compounds and sometimes leak, therefore loss of valuable volatile compounds can occur – leading to potential issues in the standardization between studies utilizing breath analysis (1–4). Our ReCIVA® Breath Sampler was designed with these standardization challenges in mind and will be discussed as an example of how breath can be collected.
Collecting a breath sample is as simple as exhaling into the device, and the compounds contained in the breath will be captured and concentrated by four independent adsorbent tubes. The device has built-in carbon dioxide and pressure sensors that monitor and learn the breath patterns of each subject in real-time, the pumps can be set to only draw air into the cartridges at the right times to collect from specific breath fractions, from deep within the lungs, the upper airways or from the throat and mouth. Therefore, depending on disease proximity and the associated volatile compounds, breath fraction collection can be accommodated. This means that depending on which volatile compounds are of interest, i.e. the compounds originating from deeper within the body than the immediate respiratory tract, the breath from the deeper parts of the lung can be collected only.
During breath collection, contaminant compounds present in the ambient air can cause analytical problems. This is because the abundance of these environmental chemicals can be much higher than the endogenously, or microbiota-originating volatile chemicals in the breath. This is a particular challenge of the entire biomarker field, whereby informative compounds are very low in concentration. To minimize the potential contribution of contaminant compounds, our CASPAR® Portable Air Supply is a specially developed device that provides a consistent airflow into the ReCIVA device for participants to inhale. Since different sampling locations also have different environmental volatile compounds, it makes it harder to compare breath samples between trial sites.
This source of bias can be significantly reduced by collecting a “blank” sample of the ambient air alongside where the breath samples are collected. These are referred to as a “breath or blank” (BoB) study designs, and enable more accurate reporting on the compounds that are endogenously or microbially originating from deeper within the body in exhaled breath as opposed to ambient volatile compounds in the ambient air that were inhaled.
How are breath samples analyzed?
Once a breath sample has been collected, there are many ways in which it can be analyzed. The methodology that can produce high-confidence data utilized at Owlstone Medical is Gas Thermo-Desorption Gas Chromatography Mass Spectrometry (TD-GC-MS).
The coupling of gas chromatography (GC) to mass spectrometry (MS) is the most versatile and sensitive method that is considered the gold standard for analyzing volatile compounds within exhaled breath. The latest HRAM GC-MS devices (such as GC-Orbitrap™) are used at Owlstone as they have the resolution to reliably identify individual compounds within complex mixtures. They also offer dynamic ranges that span the range of volatile compounds in breath alongside uniquely sensitive detection, in the parts per trillion (ppt) range, across the breadth of detectable compounds. An optimized HRAM GC-MS system can detect hundreds of compounds in a breath sample and demonstrate high consistency in detecting the same compounds across samples. They can also be further adapted and iteratively optimized based on existing results and biological insights to enhance the detection of compounds. For example, different types of GC columns can be used and adjusted to capture compounds that might have been omitted otherwise due to their chemical properties.
The first step of sample processing is thermo-desorption (TD), whereby an adsorbent material is used to collect volatile compounds contained within the breath. The thermal desorption sample introduction equipment includes a cold trap, which has the same sorbent material as the sample tubes, which enables the concentration of volatile compounds. The adsorbent is then heated, causing the adsorbent to release the volatile chemicals for GC-MS for separation, identification, and quantification. GC can be used to separate the compounds from a mixture such as breath and is optimized for the retention and peak quality of the VOCs detectable in each breath sample. After GC, mass spectrometry (MS) is used to measure the mass-to-charge ratio of ions, resulting in a dataset of mass spectra that plot intensity as a function of the mass-to-charge ratio. This leads to a series of peaks that have certain features and quantities depending on what the compound was. This resulting dataset can be processed to optimize and clean up the signals to best allow for the next step of identifying the compounds within the breath based on the characteristic features of the GC-MS data.
Once the GC-MS data has been collected and processed to correct for standard QC checks (such as misalignment of peaks), the peaks can be compared against reference libraries to identify the compounds. For completely accurate identification, this requires a comparison to purified chemical standards that have been analyzed and processed using the same instrumentation and methodology. We have recently announced the launch of our Breath Biopsy VOC Atlas, a catalog of validated and quantitated volatile compounds found in exhaled breath.
The Atlas has been built through detailed analysis of breath samples representing a diverse mix of ages and ethnic backgrounds to identify and quantify commonly seen chemicals originating from breath. At over 150 chemicals and growing, the Breath Biopsy VOC Atlas represents the largest available database of identified VOCs on breath, their normal levels, and biological interpretation. The discovery of biomarkers in breath has traditionally been performed using untargeted methods, which look at the differences between levels of chemicals in large patient populations. This approach has significant disadvantages including high cost, low throughput, and misidentified compounds due to the lack of a comparison standard. A superior approach involves performing biomarker studies starting from a set of compounds of interest that are known to be detectable in breath, however, the list of reliable and validated chemicals for breath as a biological matrix is very limited. The Atlas was designed to address this problem, and will form the basis of future breath biomarker discovery at Owlstone.
Now that we have covered the workflow behind breath collection and analysis, our next and final blog in this introductory series will focus on some case studies of disease areas where breath analysis has been used in the research efforts, and what successes have happened.
Analysis of volatile compounds in exhaled breath, or those emitted from in vitro samples can an incorporated in a variety of innovative ways in your research. If you are interested in finding out how you could incorporate breath analysis in your research, please do not hesitate to contact us.
References
- Groves WA, Zellers ET. Investigation of organic vapor losses to condensed water vapor in Tedlar bags used for exhaled-breath sampling. Am Ind Hyg Assoc J. 1996 Mar;57(3):257–63. DOI: 10.1080/15428119691014981
- Sulyok M, Haberhauer-Troyer C, Rosenberg E, Grasserbauer M. Investigation of the storage stability of selected volatile sulfur compounds in different sampling containers. J Chromatogr A. 2001 May 11;917(1–2):367–74. DOI: 10.1016/s0021-9673(01)00654-9
- Steeghs MML, Cristescu SM, Harren FJM. The suitability of Tedlar bags for breath sampling in medical diagnostic research. Physiol Meas. 2007 Jan;28(1):73–84. DOI: 10.1088/0967-3334/28/1/007
- Harshman SW, Pitsch RL, Davidson CN, Lee EM, Scott AM, Hill EM, et al. Evaluation of a standardized collection device for exhaled breath sampling onto thermal desorption tubes. J Breath Res. 2020 May 27;14(3):036004. DOI: 10.1088/1752-7163/ab7e3b