LuCID: Lung Cancer Indicator Detection
A multi-centre prospective trial for lung cancer screening
OVERVIEW
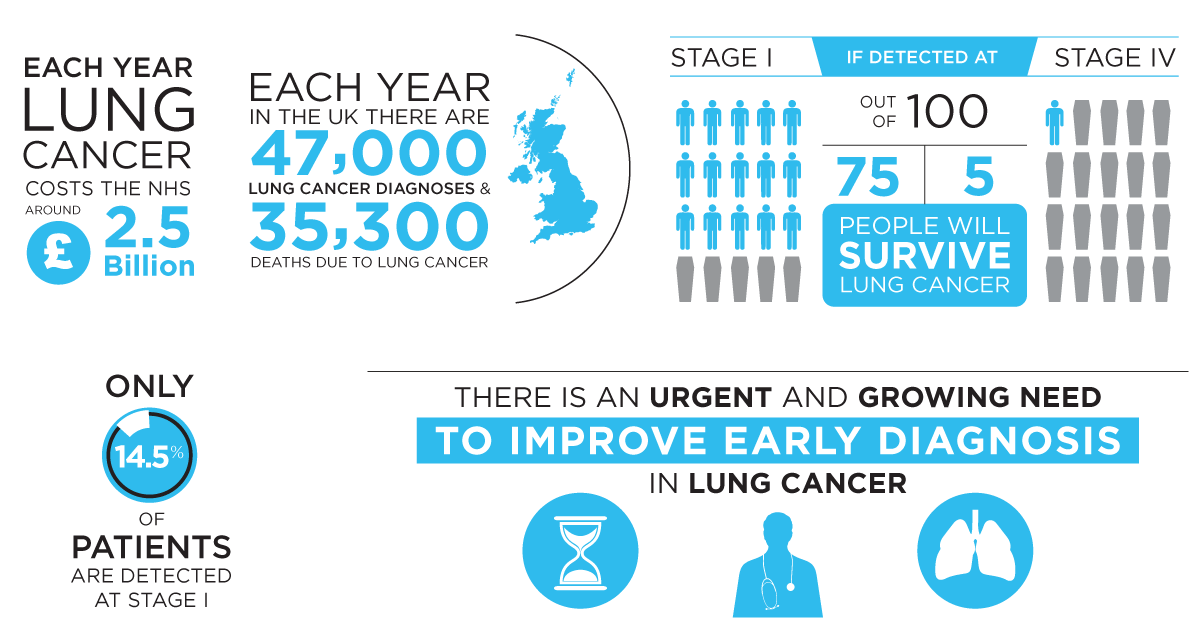
Early detection saves lives
Funded by an NHS development contract, Owlstone Medical is conducting research into the diagnosis of lung cancer by measuring volatile organic compounds (VOCs) in patients’ exhaled breath. Phase I of the project is complete and Phase II is underway.
We established that Owlstone Medical’s FAIMS technology can detect biomarkers with a promising sensitivity: detecting at threshold concentrations ten times lower than previously reported. An initial analysis identified that successful introduction of a lung cancer screening programme using ReCIVA and FAIMS could could save approximately 3,200 lives per year and £82M in treatment costs in the UK alone.
The second phase of the study is funded by a £1.1m SBRI grant from the UK National Health Service and aims to validate detection of lung cancer biomarker by FAIMS in clinical practice. The final phase of the study will be a population based screening trial. You can read the LuCID infographic here.
TAKING PART
Taking part in the clinical trial
To be able to take part in this study the patient must be under clinical suspicion of having lung cancer, be aged 18 or older and agree to provide written consent to taking part in the clinical trial. Patients who attend a clinic that is registered for the trial may be invited to take part; it is not possible to volunteer for this study.
2015
Start of the LuCID clinical trial
1760
Total numbers of participants to date
26
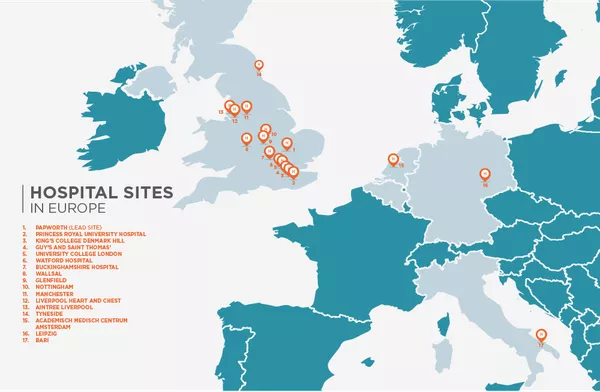
Total number of clinical sites the clinical trial is taking place in
4000
Patients taking part

What to expect if you are selected
A nurse will explain the study to you and answer any of your questions. If you are happy to proceed, the nurse will fill in a questionnaire with information about you and your health and take a sample of breath using Owlstone Medical’s ReCIVA breath sampler. This sample will be analysed by Owlstone Medical in its Cambridge Breath Biopsy Laboratory. These results, along with other information that we get from your doctor such as whether the tumour is cancer, will be used to develop a new test to screen for lung cancer.
STUDY PROTOCOL
Summary of the protocol
- DESIGN
An international multi-centre prospective study designed to evaluate breath analysis in a clinical setting
- PRIMARY OUTCOME
To evaluate the diagnostic accuracy (sensitivity and specificity) of a breath test for lung cancer
- PATIENT ELIGIBILITY
Patients, aged 18 or older, in secondary care with a clinical suspicion of lung cancer
- WHAT TO EXPECT
After a briefing and agreeing to take part a nurse will take a sample of breath using ReCIVA
- BREATH ANALYSIS
Analysed using Owlstone Medical’s propriety Field Asymmetric Ion Mobility Spectrometry technology
- DATA ANALYSIS
The test uses patient information and breath biomarkers to predict whether a patient has lung cancer
STUDY TEAM
The far reaching scale of the clinical trial
Led by Dr Robert Rintoul at Papworth Hospital near Cambridge UK, the study investigators are recognised experts in lung cancer at some of Europe’s leading hospitals and clinics. Clinical sites across 5 different countries are participating in the clinical trial.