FAIMS could be a non-invasive solution for pancreatic cancer detection
Urinary VOCs could be used to discriminate healthy individuals from pancreatic cancer patients
| Publication information: Arasaradnam et al., Non-invasive Diagnosis of Pancreatic Cancer Through Detection of Volatile Organic Compounds in Urine Gastroenterology, 2017. 154 (3), e16-e17. DOI: 10.1053/j.gastro.2017.09.054
Disease Area: Pancreatic Cancer Application: Early Detection Sample medium: Urine Analysis approach: FAIMS Products: Lonestar® VOC Analyzer, ATLAS Headspace Sampler Summary:
|
Worldwide more than 330,000 people die from pancreatic ductal adenocarcinoma (PDAC) every year1, and it is predicted to become the second leading cause of cancer-related deaths by 20302. Only 1% of people diagnosed with PDAC survive more than 10 years – a figure which has changed little in the last 40 years1.
Most patients (around 80%) have already reached the later stages of the disease by the time they are diagnosed, which is why pancreatic cancer has one of the lowest survival rates of all cancers2. When detected at an early stage, survival dramatically increases3. However, early detection of PDAC is challenging due to lack of specific clinical symptoms, and limitations of current diagnostic techniques based on imaging or serum biomarkers. Determination of accurate, non-invasive biofluid-based biomarkers for detection of the early-stage disease could result in potentially dramatic improvements in survival.
A clinical study published by Arasaradnam et al.4 used Owlstone Medical®’s FAIMS technology to detect urinary volatile organic compounds (VOCs), and use the VOC profile to discriminate between healthy individuals and patients with PDAC.
VOCs in biofluids such as urine originate from metabolic processes, as well as from the body’s microbiome and environment. The authors of this study postulate that altered cell physiology and/or microbiome in patients with PDAC provide a characteristic VOC profile detectable from urine.
The authors used a Lonestar VOC Analyzer fitted with an ATLAS Headspace Sampler to analyze 5 ml urine samples from 81 patients with stage I – IV PDAC (Table 1) as well as 81 healthy individuals.
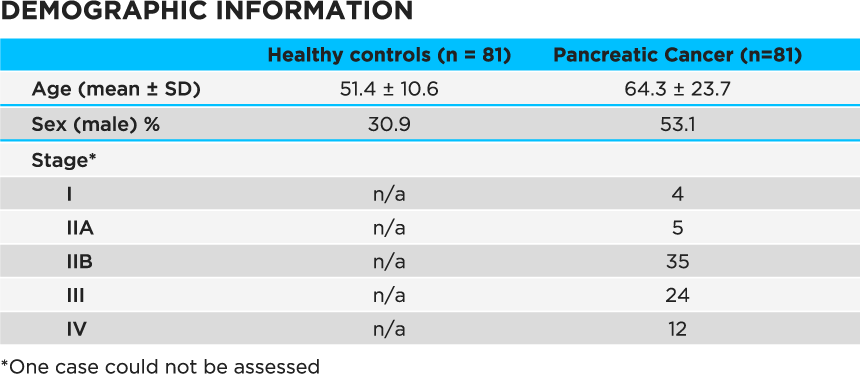
Table 1 Demographic information for study subjects. Age was not deemed to be a confounder based on previous published work – see supplementary material4.
Using an established data processing pipeline5, features from the urine sample FAIMS spectra were identified. Different classification algorithms were then used to calculate clinically relevant values such as AUC (area under receiver operating characteristic curve), sensitivity and specificity.
Urine VOCs could discriminate between PDAC and healthy individuals with sensitivity of 0.91 and specificity of 0.83, and with an AUC of 0.92 (Figure 1c). Using the same analysis pipeline, PDAC patients with stage I/II disease could be distinguished from healthy individuals with sensitivity of 0.91 specificity of 0.78, AUC of 0.89 (Figure 1d). The VOC profile could also differentiate between stage I/II patients and patients in the later stages of the disease (stage III/IV) with sensitivity of 0.82, specificity of 0.89, AUC of 0.92 (Figure 1e).
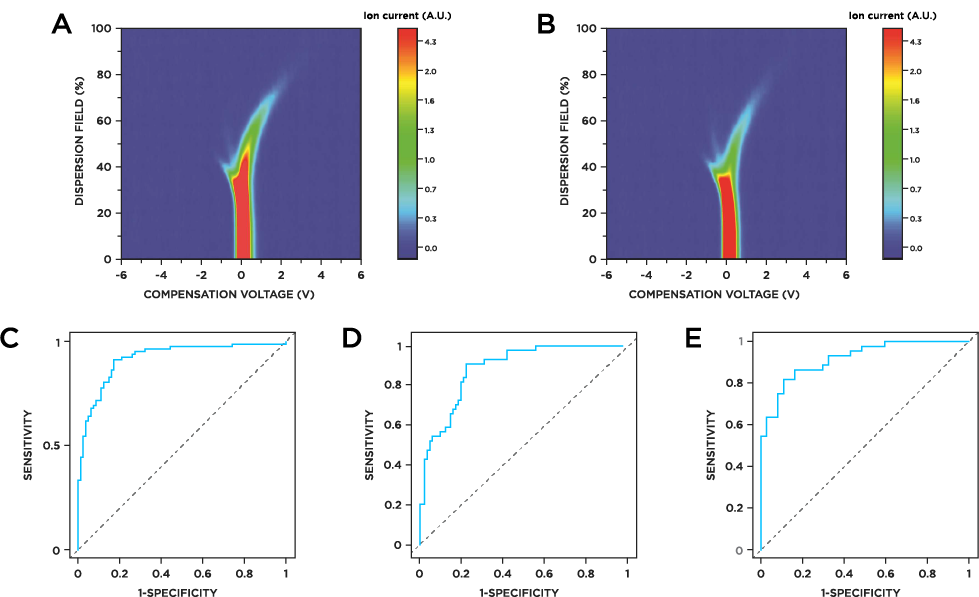
Figure 1a) FAIMS spectrum of urine sample from healthy control. b) FAIMS spectrum of urine sample from PDAC patient. c) Receiver Operating Characteristic (ROC) curve showing performance of VOCs in differentiating healthy individuals from PDAC, AUC = 0.92 (CI: 0.88-0.96), sensitivity = 0.91 (CI: 0.83-0.96), specificity = 0.83 (CI: 0.73-0.9. d) Differentiation of healthy individuals from early stage I/II PDAC AUC = 0.89; CI: 0.83-0.94, sensitivity = 0.91 (CI: 0.78-0.97), specificity = 0.78 (CI: 0.69-0.86). e) Differentiation of early stage PDAC (I/II) from advanced stage PDAC (III/IV); AUC = 0.92 (CI: 0.86-0.97), sensitivity = 0.82 (CI: 0.67-0.92), specificity = 0.89 (CI: 0.75-0.97).
This proof of concept study demonstrates for the first time that FAIMS analysis of urine specimens can be used to discriminate healthy individuals from PDAC patients through the detection of VOCs using a non-invasive biofluid sample. It also shows that healthy individuals can be separated from those in the early stage of the disease, and that early stage PDAC can be separated from more advanced stages of the disease.
References
- Cancer Research UK
- Rahib et al., Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States, Cancer Research, 2014. DOI: 10.1158/1078-0432.CCR-14-2467
- Radon et al., Identification of a Three-Biomarker Panel in Urine for Early Detection of Pancreatic Adenocarcinoma, Clinical Cancer Research, 2015. DOI: 10.1158/1078-0432.CCR-14-2467
- Arasaradnam et al., Non-invasive Diagnosis of Pancreatic Cancer Through Detection of Volatile Organic Compounds in Urine, Gastroenterology, 2017. 154 (3), e16-e17. DOI: 10.1053/j.gastro.2017.09.054
- Covington et al., The application of FAIMS gas analysis in medical diagnostics, Analyst, 2015. DOI: 10.1039/c5an00868a
