Breath Biopsy for Identification of VOC Biomarkers for Liver Cirrhosis
Published on: 13 Feb 2023
We’ve been working to develop a breath test for liver disease for a number of years. Liver disease is one of the areas that we’re expectant of realizing our twin aims of saving lives and healthcare spending. Our research is ongoing as we continue to work towards producing a non-invasive breath test that improves our ability to detect, stage, and monitor progression or recovery from liver disease.
Read the Full Paper READ THE LATEST PRESS RELEASE
Lifestyle related factors have led to a rapid rise in the prevalence of liver diseases, with as many as 3 in 10 adults affected in some countries, alongside an increasing number of children. However, progression of liver disease is often asymptomatic, leading to more than 50% of cases being diagnosed at advanced stages.
Early detection of liver diseases, such as non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH) and more advanced cirrhosis, is a significant clinical challenge, with the gold standard still tissue biopsy, despite recent developments in both imaging techniques and blood biomarkers. A non-invasive approach that can be offered as part of a screening program represents an ideal solution and we are developing Breath Biopsy® to meet this need.
In our previous study, Ferrandino et al. used Breath Biopsy® OMNI® to compare the breath of healthy volunteers to that of patients with cirrhosis, at a non-severe stage, with an analytical focus on limonene. Limonene is a VOC that had already been identified in the literature as having a strong association with liver diseases. This study provided proof of principle that breath limonene levels can differentiate controls from subjects with cirrhosis. Additionally, breath limonene levels correlated with blood biomarkers of liver function.
Despite this qualified success, we don’t believe that the complexity of the liver’s metabolic pathways can be accurately represented by limonene alone, as a single biomarker. We have therefore followed up our work on limonene with untargeted analysis. The new paper from Ferrandino et al. discusses how we have now used Breath Biopsy OMNI global VOC analysis to perform an untargeted analysis of the VOCS on the breath of patients with cirrhosis, compared to controls in order to identify additional potential disease-related biomarkers.
46 subjects with cirrhosis (disease severity classified according to the Child-Pugh system), and 42 controls provided breath samples via the ReCIVA® Breath Sampler, part of the Breath Biopsy Collection Station. 29 VOCs exhibited statistically significant difference between the cirrhosis group and controls. The top four performing compounds for differentiating these groups were 2-pentanone, eucalyptol, limonene and dimethyl selenide. Interestingly, these other compounds showed a correlation with blood metrics of liver function, and their combination, in a classification model, provided a better classification performance compared to each single compound.
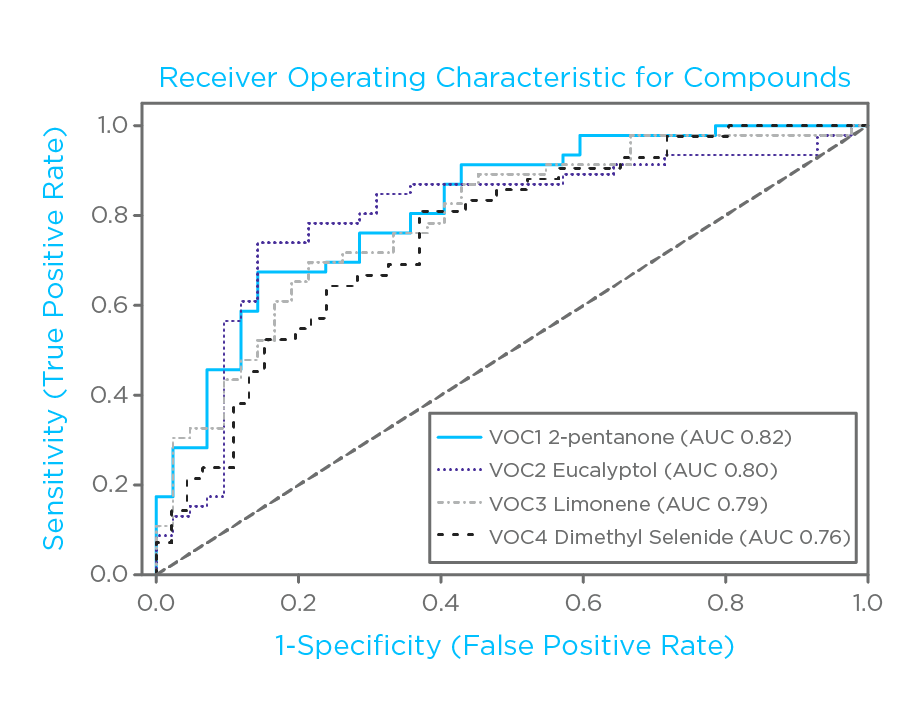
Figure 1: Receiver originating characteristic plots of the four top single VOCs comparing cirrhosis vs controls.
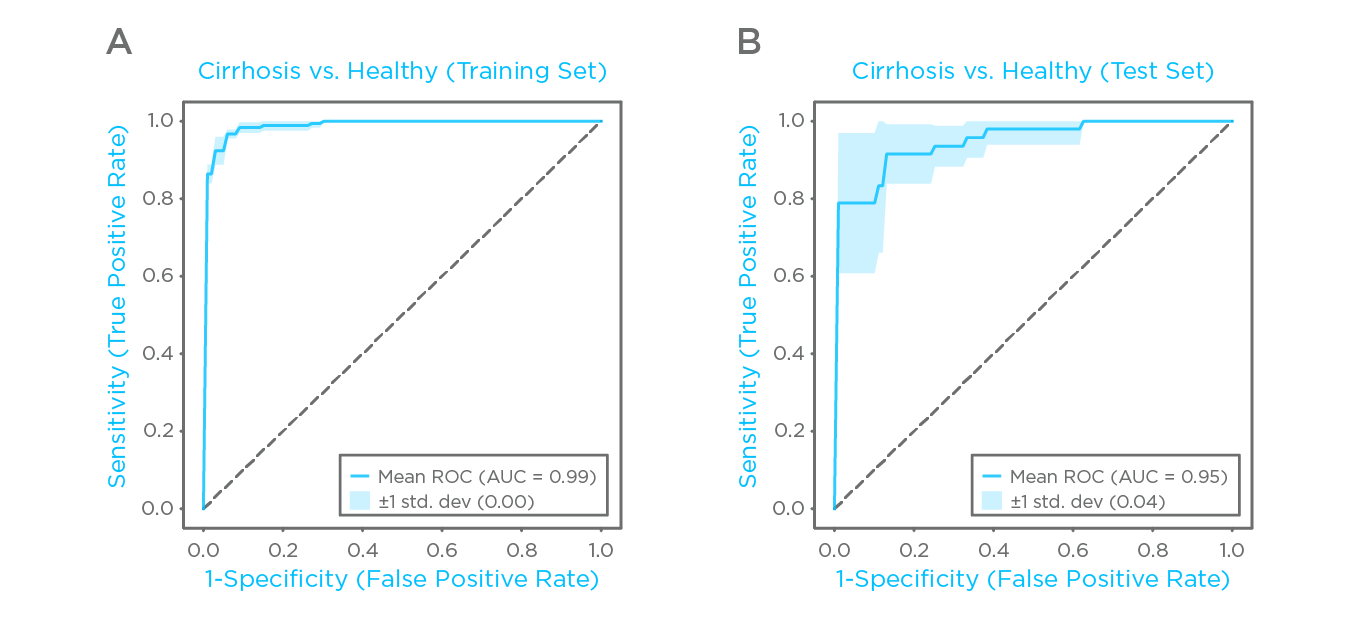
Figure 2: Classification performance of combined VOCs. (A) ROC plot and confidnece interval obtained for the training set. (B) ROC plot and confidence interval obtained for the test.
A model based on combinations of the best performing VOCs was created and cross validated using a data split for training/testing of (70%/30%). On the training sets, the model returned an average area under the ROC curve (ROC-AUC) of 0.99 ± 0.00 (Fig. 4A), and on the test sets, an average ROC-AUC of 0.95 ± 0.04 (Fig 4B). Further analysis explored the correlation between identified breath compounds and other established markers of hepatic function, as well as looking at the effect of cirrhosis severity on exhaled VOCs.
Using rigorous the sampling procedures associated with Breath Biopsy OMNI for this study represented a substantial advance over similar prior studies. OMNI is an end-to-end optimized service for consistent breath analysis. In this instance employing OMNI meant, firstly, we purified inhaled air to minimize environmental contaminants. Secondly, the volume of sampled breath or blanks was standardized and closely monitored. And thirdly, that multiple blanks were collected alongside each session, allowing experimental discrimination of contaminants from on-breath VOCs. Taken together these measures have provided us with a higher degree of confidence discerning on-breath VOCs from environmental contaminants.
We’re continuing our research in this area, in an attempt to validate our in vivo findings reported in the above paper. Using the Breath Biopsy OMNI Assay, we have now performed in vitro experiments using cultured hepatocytes that have allowed us to get closer to demonstrating mechanistic links between liver disease biology and our biomarker candidates.
Dr Giuseppe Ferrandino presented some of our in vitro findings at our recent webinar on ‘Identifying Translational Biomarkers using Breath Biopsy in vitro Headspace Analysis’ which you can now watch on-demand.
WATCH THE LIVER DISEASE IN VITRO WEBINAR BREATH BIOPSY OMNI WHITEPAPER