Progressing from Biomarker Discovery to Breath Biopsy Tests
Human breath has great potential as a source of information on human health and disease. There are many methods available to collect and analyze breath and, as we have previously discussed, each has their own strengths and weaknesses when it comes to clinical applications. We can support you to design and develop a appropriate breath testing methods that are fit for purpose and address key clinical unmet needs.
Published on: 11 Jan 2022
Human breath has great potential as a source of information on health and disease. There are many methods available to collect and analyze breath and, as discussed in previous blogs, each have their own strengths and weaknesses when it comes to clinical applications. We can support you to design and develop appropriate breath testing methods that are fit for purpose and address key clinical unmet needs.
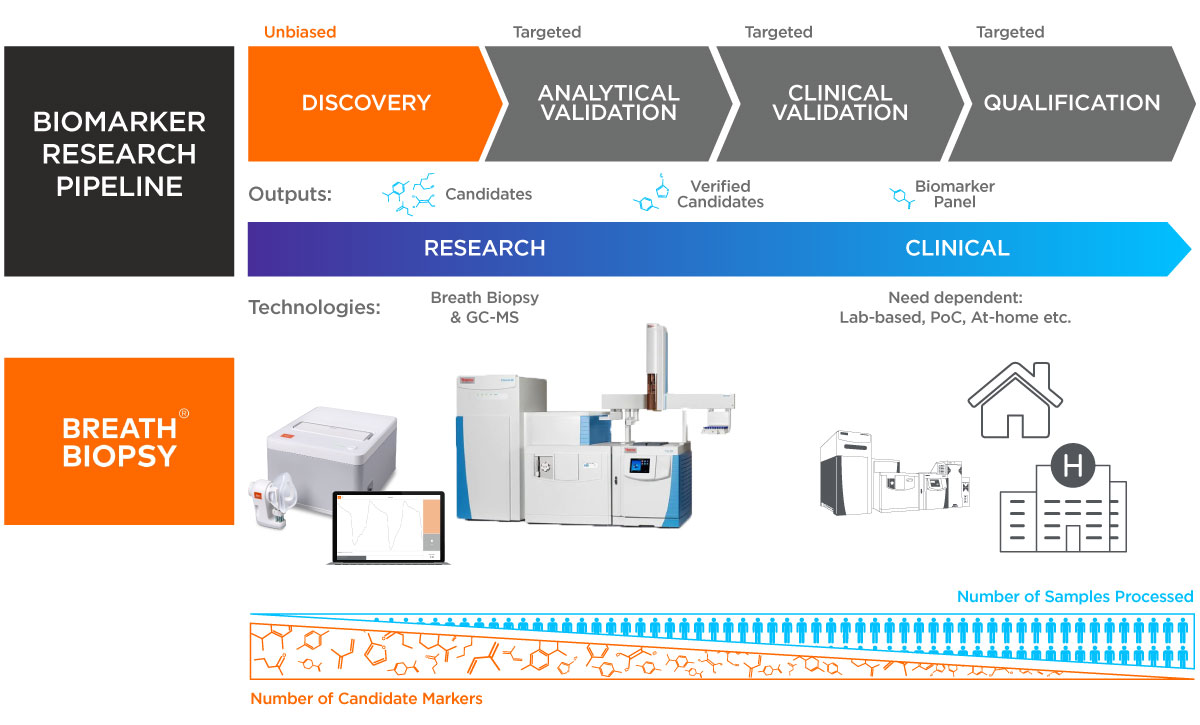
In developing Breath Biopsy®, we have considered which methods are most appropriate for different stages in the process of biomarker development. In our view, during the early stages of biomarker discovery and validation, it is most important to use techniques that maximize the opportunity for biomarker detection. This means having an ability to detect a broad range of relevant volatile organic compounds (VOCs), with high sensitivity and a wide dynamic range. It is for this purpose that we created Breath Biopsy OMNI combining our Breath Biopsy Collection Station, including our ReCIVA® Breath Sampler, and high-resolution accurate mass (HRAM) gas chromatography-mass spectrometry (GC-MS).
For downstream routine application of validated biomarkers in the clinic, factors such as speed, ease of use, and cost become more critical, and methods should be tailored to the specific clinical needs of the associated test. With over 15 years of experience in easy-to-use, portable, and precise chemical detection technologies, Owlstone Medical is well-placed to help with both the development and subsequent deployment of these clinical tests.
Why is identifying breath biomarkers important?
Identification of candidate biomarkers is an essential step towards biomarker and clinical validation, required for subsequent regulatory approval. Knowing the identity of biomarkers allows us to develop bespoke, high-accuracy breath tests suitable for use in clinical settings. Since biomarkers are mainly used for decision-making in clinical pathways, it is critical to follow pilot studies with studies that validate clinical performance in the intended for use population.
Techniques that can characterize and identify biomarkers provide connections between biomarkers and biology that can inform test development and support successful applications for regulatory approval. For example, biomarker standards are needed to develop and test point-of-care devices that are reliable and able to provide consistent results in larger studies. This, however, relies on high-confidence biomarker identification, and currently many studies only use tentative identities based on generic compound libraries.
Questions to consider when designing studies
OMNI: GC-MS to detect and identify novel breath biomarkers

While any single GC-MS method cannot be relied upon to detect all possible compounds in a sample, it can detect large numbers of compounds from a single sample. Breath Biopsy OMNI, for example, has been optimized for the detection of on-breath compounds, volatile chemicals that are detected in breath samples and are likely to have relevance to human biology. We have developed OMNI in this way to provide a broad-ranging service for global breath biomarker analysis that can be applied to a range of research questions and clinical needs.
More about Breath Biopsy OMNI DOWNLOAD OMNI WHITEPAPER
At present, few breath biomarkers have been identified and suitably validated clinically and this is why novel biomarker analysis using GC-MS continues to be a key focus of our work. We have designed OMNI with biomarker development in mind, and we are able to support high-confidence biomarker identification as an extension to OMNI studies.
Breath Biopsy Tests need to be appropriate to the clinical needs
When it comes to developing biomarkers for Breath Biopsy Tests for clinical use, we believe that the type of test and the methods used should be suited to the application. We are prepared and equipped to support various eventualities for late-stage biomarker development and clinical launch.
There are a number of factors that could affect the final form of a Breath Biopsy Test, including:
- Unmet clinical need – Does the test need to be performed in the hospital? How quickly are the results needed? How often do people need testing? How many people need to be tested?
- Clinical performance – How sensitive and specific does the biomarker/test need to be to impact the clinical outcome for the patients?
- Biomarker nature – The chemical properties of the biomarker(s) will limit the possible detection methods that can be used.
The above considerations will influence the final form of a breath test, from point-of-care devices that can provide rapid results (akin to COVID-19 lateral flow testing) in a near-patient format, through to laboratory-based tests where samples are sent for offline analysis in a central laboratory (as is typical for blood tests). The rise of Digital Health programs, further accelerated by the pandemic, is a major driver for pharmaceutical companies to develop biomarker modalities (such as breath) that can be translated into a point-of-care diagnostic and deployed in primary care or even home-based setting.
Through our work on FAIMS, we already have extensive experience in developing and releasing sensor-based chemical detection systems for various industrial applications. More recently, through our partnership with Functional Gut Diagnostics, we have started providing lab-based analysis for at-home hydrogen methane breath tests (HMBT) supporting the diagnosis of multiple digestive health issues. As such, we have skills and experience relevant to support the clinical development and implementation of breath tests of various forms.
If you are seeking non-invasive biomarkers and would like to find out more about breath and the potential for breath-based clinical tests for early detection, precision medicine, drug development, and other applications, please contact us.
