The Early Detection of IPF Using Breath Analysis
Published on: 15 Dec 2023
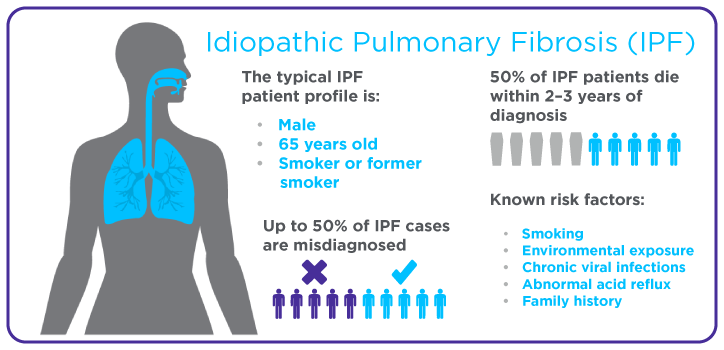
Interstitial lung disease (ILD) is a group of chronic lung conditions that cause a thickening or scarring of lung tissue, known as fibrosis, because of lung inflammation. Pulmonary fibrosis is one of the most irreversible forms of fibrosis across different organs, despite the lungs’ high capacity to repair itself. The scar tissue in the lungs can make it very difficult to breathe. Idiopathic pulmonary fibrosis (IPF) is the most common and fatal form of pulmonary fibrosis, and it can lead to secondary respiratory failure, with a median survival rate of 3-5 years from diagnosis (1).
Unfortunately, treatments for IPF will not cure the disease, but they can make it easier for patients to breathe. There are currently two drugs that are approved to treat IPF – nintedanib and pirfenidone. These medications block the process in the lungs that leads to scarring and therefore keep IPF from progressing. However, these medications are only effective if they are used in patients who have been diagnosed early (2). Patient surveys and studies have shown a significant delay in the diagnosis of IPF, as the time from onset of symptoms until a final diagnosis is made, can take between 7 months and over 2 years (3).
This delay could be because IPF is hard to diagnose as its symptoms are similar to other lung conditions, such as chronic obstructive pulmonary disease (COPD) (4). Early detection methods are needed to improve the treatment outcomes of IPF patients; analysis of breath could provide a non-invasive method to diagnose IPF early.
Compounds in the breath that are associated with IPF.
Volatile organic compounds (VOCs) are found in various excreted biological materials and can serve as indicators of metabolic shifts triggered by IPF, including inflammation. The metabolic by-products from these processes are released into the bloodstream and reach the alveoli, renal tubules, and gastrointestinal tract where they can be excreted in breath, urine, and feces (5). In IPF cases, these VOCs can be released directly into the breath as it is in constant contact with the lungs, and therefore VOCs are promising targets for new biomarkers for IPF screening and diagnosis.
A systematic review found twenty biomarkers found in the breath that can discriminate between IPF patients and healthy controls (6).
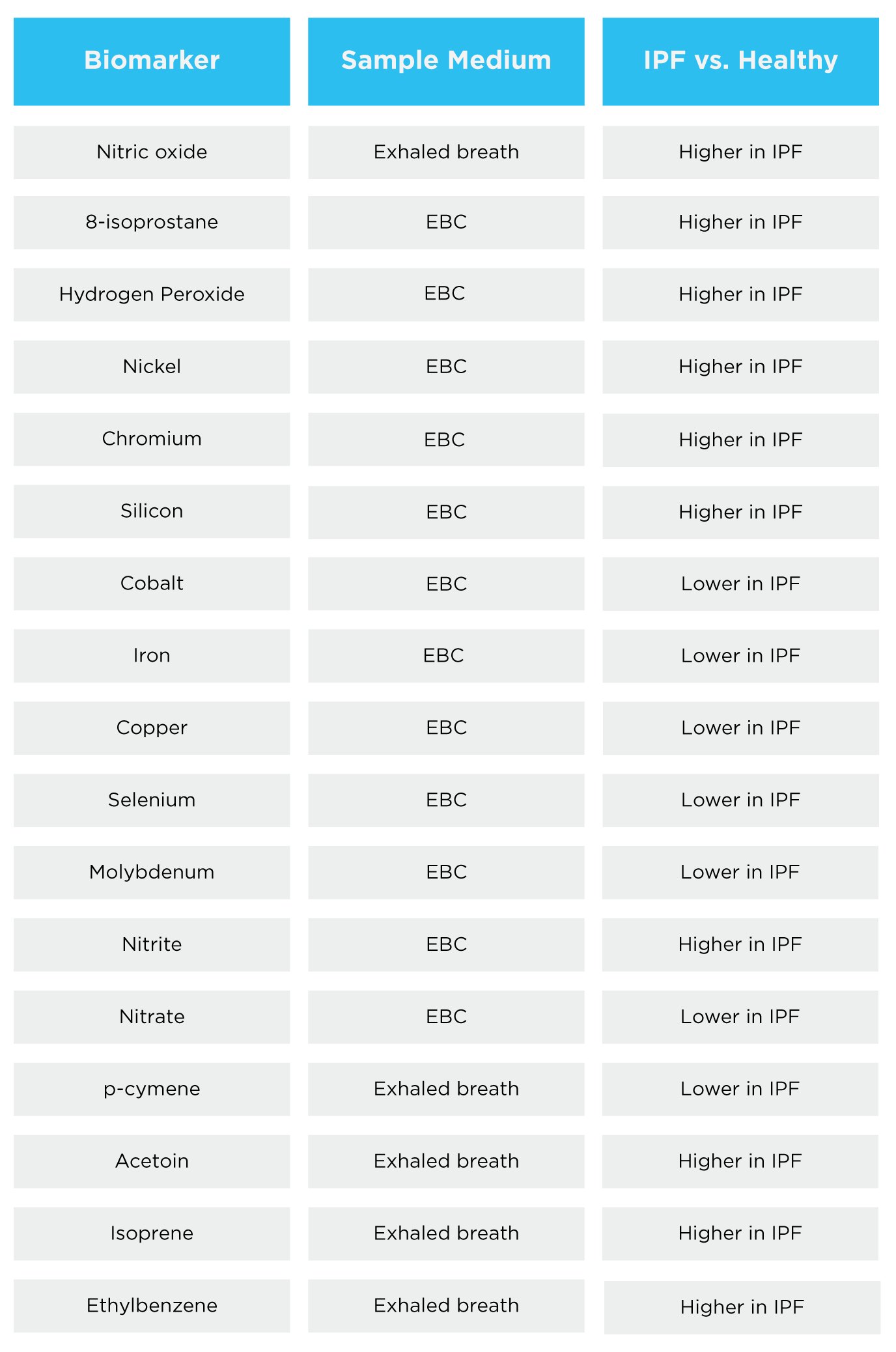
Table 1 – Biomarkers that have been reported to be able to discriminate between IPF patients and healthy controls. EBC = exhaled breath condensate (6).
Exhaled nitrous oxide (NO) is a breath biomarker that is easily measurable and is used confidently in asthma diagnosis. NO is a free radical that is implicated in a variety of pathophysiological processes, and it has been suggested that NO may increase the expression of pro-fibrotic mediators in lung fibrosis (6). NO is elevated in the breath of IPF patients compared to healthy controls (6). Fractional exhaled nitric oxide (FeNO) is the most common method for measuring NO in breath, however, the flow rate used in this test can only measure the higher lung fraction of airway NO and not the levels produced in the lower fraction of the lungs by the alveoli (7).
Our Breath Biopsy Collect software alongside our ReCIVA breath collection device can overcome this issue and be used to analyze specific breath fractions from the lungs. This flexibility can allow the selective capture of VOCs originating from different depths of the lungs. Breath samples from a specific fraction of the lungs close to the alveolar surface can therefore ensure that collected air is enriched with VOCs produced by the fibrotic processes occurring in IPF.
Oxidative stress is a possible mechanism suggested to be involved in the fibrotic process. VOCs such as hydrogen peroxide (a reactive oxygen species) and 8-isoprostane (a compound formed by lipid peroxidation) are elevated in patients with IPF (8). P-cymene shows great promise as a clinical biomarker for IPF as it displays a strong correlation with other markers of disease severity in IPF patients. P-cymene is thought to have antioxidant properties and therefore the reduced levels seen in IPF patients would correlate with the theory of oxidative stress being a cause of fibrosis in the lungs (9).
The identification and understanding of VOCs in breath as potential biomarkers for the early detection of IPF represents a promising avenue of research. Enhanced research efforts in this area hold the potential for earlier detection, improved treatment outcomes, and ultimately a reduction in the number of deaths caused by IPF worldwide.
Breath analysis can support IPF research.
VOCs that originate or are elevated from IPF can readily enter our lungs and be exhaled from the body in breath. These compounds can be analyzed in breath and can provide insight into the development and prognosis of IPF. As breath is a sampling medium that is in direct contact with the lungs, analysis of breath can provide a better insight into the disease as opposed to blood or urine which are more indirect.
Breath is a virtually inexhaustible resource, with large volumes able to be collected at frequent intervals, meaning breath analysis can provide an almost real-time longitudinal analysis of VOCs. The non-invasive nature of breath analysis makes it more attractive than sputum samples or scans which can be uncomfortable for the patient.
We are the world leaders in breath research for the early detection of disease, which is crucial in the diagnosis of IPF. We are here to help you include breath analysis in your research. Breath Biopsy OMNI can provide everything you might need to start or continue using breath analysis, and you can receive expert advice from our team of scientists. You can cross-check your data against our VOC Atlas to better understand the baseline levels of VOCs in a healthy population and confidently validate your IPF biomarkers.
References.
- Selvarajah B, Platé M, Chambers RC. Pulmonary fibrosis: Emerging diagnostic and therapeutic strategies. Mol Aspects Med. 2023 Dec 1;94:101227. DOI: 10.1016/j.mam.2023.101227
- Ruaro B, Gandin I, Pozzan R, Tavano S, Bozzi C, Hughes M, et al. Nintedanib in Idiopathic Pulmonary Fibrosis: Tolerability and Safety in a Real Life Experience in a Single Centre in Patients also Treated with Oral Anticoagulant Therapy. Pharmaceuticals. 2023 Feb 16;16(2):307. DOI: 10.3390/ph16020307
- Hoyer N, Prior TS, Bendstrup E, Shaker SB. Diagnostic delay in IPF impacts progression-free survival, quality of life and hospitalisation rates. BMJ Open Respir Res. 2022 Jul 1;9(1):e001276. DOI: 10.1136/bmjresp-2022-001276
- nhs.uk [Internet]. 2017 [cited 2023 Dec 8]. Idiopathic pulmonary fibrosis – Diagnosis. Available from: https://www.nhs.uk/conditions/idiopathic-pulmonary-fibrosis/diagnosis/
- Strimbu K, Tavel JA. What are Biomarkers? Curr Opin HIV AIDS. 2010 Nov;5(6):463–6. DOI: 10.1097/COH.0b013e32833ed177
- Hayton C, Terrington D, Wilson AM, Chaudhuri N, Leonard C, Fowler SJ. Breath biomarkers in idiopathic pulmonary fibrosis: a systematic review. Respir Res. 2019;20:7. DOI: 10.1186/s12931-019-0971-8
- ATS/ERS Recommendations for Standardized Procedures for the Online and Offline Measurement of Exhaled Lower Respiratory Nitric Oxide and Nasal Nitric Oxide, 2005. Am J Respir Crit Care Med. 2005 Apr 15;171(8):912–30. DOI: 10.1164/rccm.200406-710ST
- Psathakis K, Mermigkis D, Papatheodorou G, Loukides S, Panagou P, Polychronopoulos V, et al. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur J Clin Invest. 2006 May;36(5):362–7. DOI: 10.1111/j.1365-2362.2006.01636.x
- de Oliveira TM, de Carvalho RBF, da Costa IHF, de Oliveira GAL, de Souza AA, de Lima SG, et al. Evaluation of p-cymene, a natural antioxidant. Pharm Biol. 2015 Mar 4;53(3):423–8. DOI: 10.3109/13880209.2014.923003