The Growth of Breath Research in Clinical Trials since 1994
Published on: 7 Dec 2018
This blog was initially published in December 2018. We aim to update it with new data on a regular basis. The last update was on 15th May 2025.
Cumulative number of clinical trials involving breath analysis
Analysis of clinical trial data uploaded to clinicaltrials.gov indicates that a total of 2103 studies have used or are using a form of breath analysis as part of their workflow (1). The number of studies incorporating breath analysis has increased every year, indicating a growing understanding of the power that breath analysis can bring to clinical trials. Exhaled breath contains thousands of compounds that have the potential to serve as biomarkers for a range of clinical use, and in a range of disease focuses.
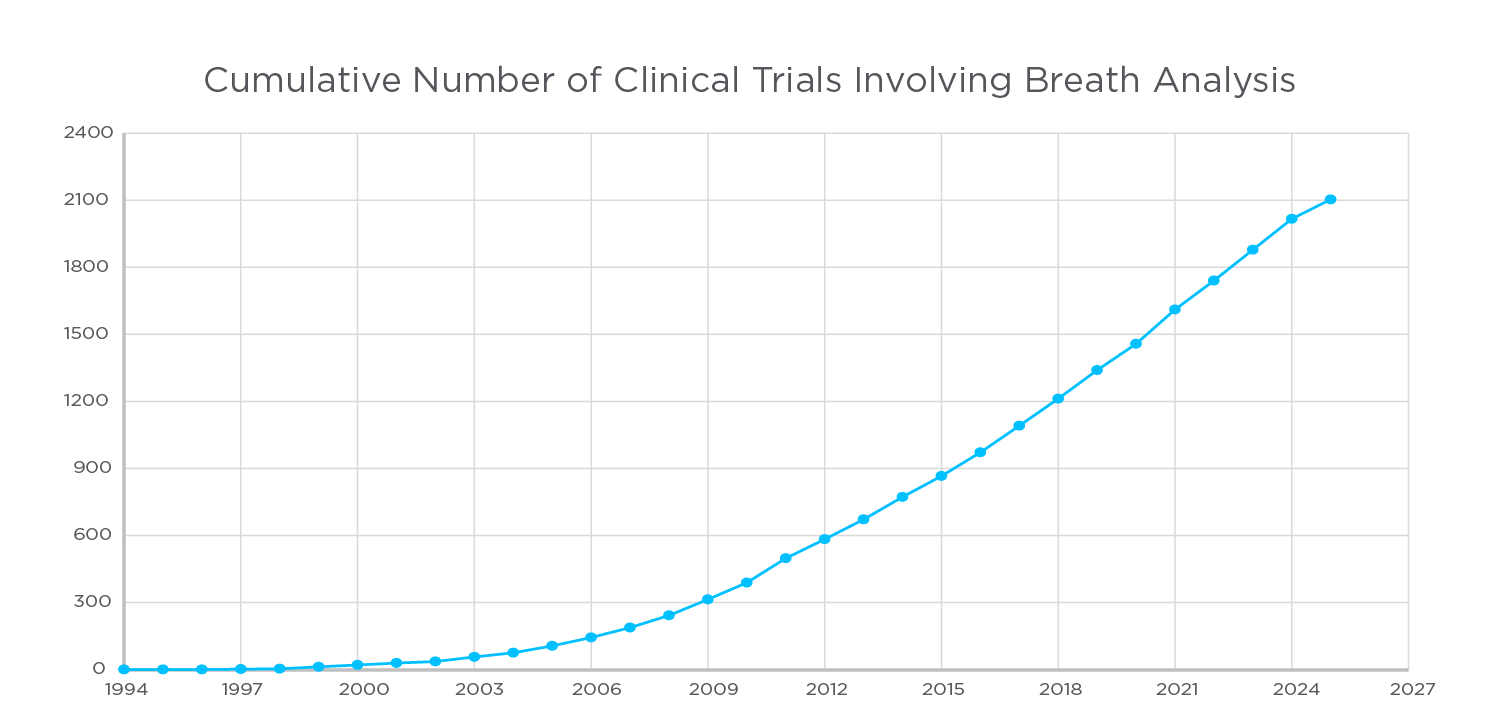
Figure 1. The cumulative number of clinical trials conducted that involve breath analysis as part of their workflow, clinicaltrials.gov was used to compile the data, with search criteria: [“breath analysis” OR “breath VOC” OR “Breath Analysis” OR “breath VOCs” OR “breath biomarker” OR “breath biomarkers” OR “breath volatile organic compound” OR “Exhaled Breath”]
Study area categorization of the 134 clinical trials in 2024 which are using exhaled breath and breath analysis
The conducted clinical trials involving breath span a broad range of research areas, and the 134 identified trials which started in 2024 were categorized into their relative fields. The criteria used to categorize the studies are outlined in the figure legend.
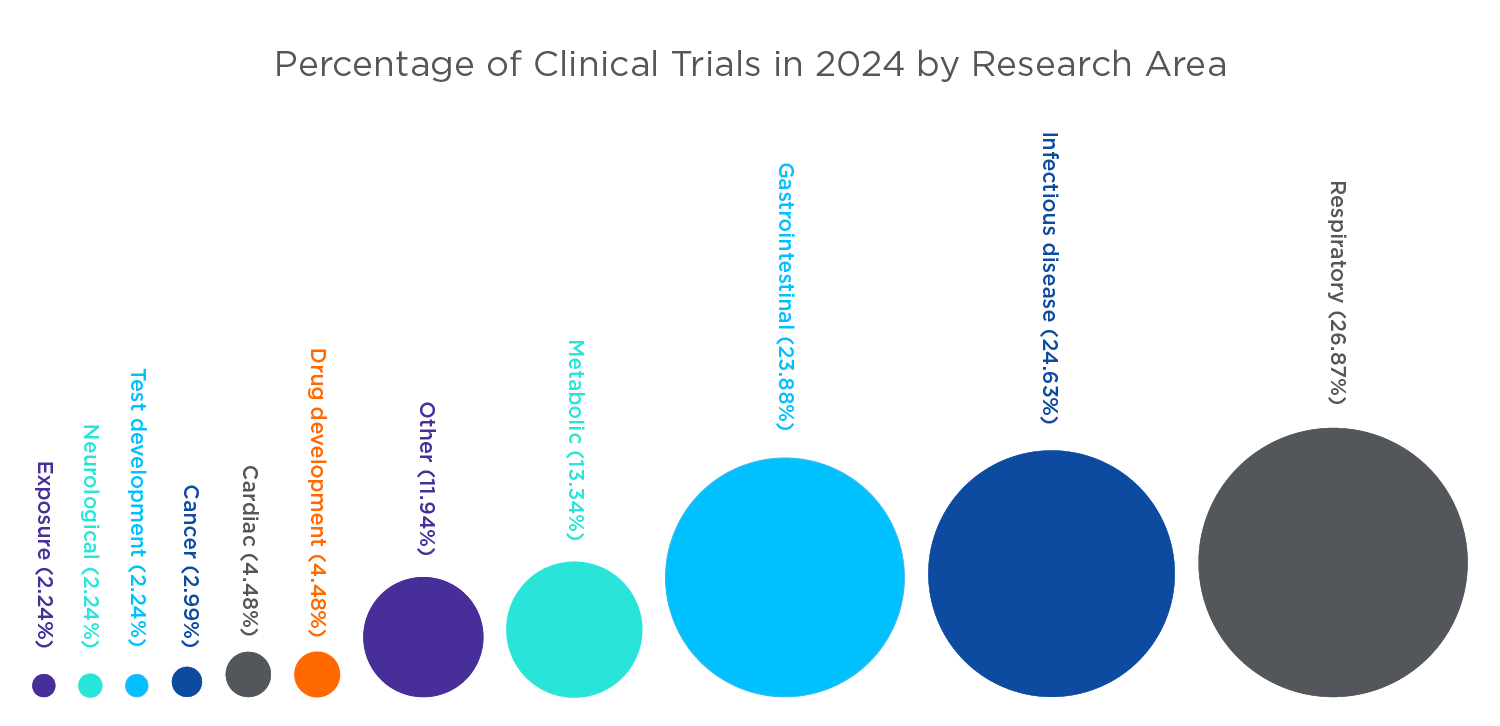
Figure 2. The categories of clinical trials in 2024 using breath analysis. The criteria for categorization were as follows: If evaluating the performance of a diagnostic test for a condition or the feasibility of a breath test – it was labelled test development. If evaluating the performance of a pharmaceutical (a drug treatment), it was labelled drug development. If the trial was evaluating a supplement or other treatment that is available over the counter (OTC), it was labelled as its area of research instead. All other trials were labelled based on the disease area the clinical trial was focused within. All data were retrieved from clinicaltrials.gov/.
From this data, breath analysis is increasingly becoming utilised in the trials of drug treatments or the development of new diagnostic tests. The most common disease area breath research is utilised within is gastrointestinal, followed by respiratory conditions. The gut microbiome is responsible for producing many of the gases and volatile compounds that can be detected in exhaled breath that are relevant for health and disease, including gastrointestinal conditions, cardiometabolic disease and cancer.
We can bring breath analysis to your clinical research.
As interdisciplinary specialists in breath, we have created Breath Biopsy® OMNI® to be the most advanced solution for reliable global breath biomarker analysis. We can support your investigation with our extensive study design, management, and data interpretation expertise.
When you work with us you also get access to our Breath Biopsy VOC Atlas. The VOC Atlas is a comprehensive catalogue of VOCs identified on exhaled human breath that will facilitate future biomarker discovery. These breath biomarkers can be used to study many disease contexts, as demonstrated by the wide variety of clinical trial areas that have been and are currently being conducted all over the world. Currently, more than 150 VOCs that have been rigorously identified and validated are registered within the ATLAS.
If you have any questions or would like more information, please do not hesitate to contact us to discuss investigating breath biomarkers for your disease of interest.
top tips for study design Selecting the right controls Standardizing your results
Breath Biopsy®
If you want to learn more about how Owlstone Medical®‘s Breath Sampling and Analysis technology and Services are being utilized in early detection and precision medicine, why not download our free ebook: Breath Biopsy: The Complete Guide?
References
1. Data from https://clinicaltrials.gov/.

