Introducing Owlstone: What do we do and how can we help?
Published on: 26 Jul 2022, under Breath Biopsy
As part of our mission to save 100,000 lives and $1.5B in healthcare costs we developed Breath Biopsy® OMNI, a platform that standardizes the collection and analysis of volatile organic compounds (VOCs) present on breath.
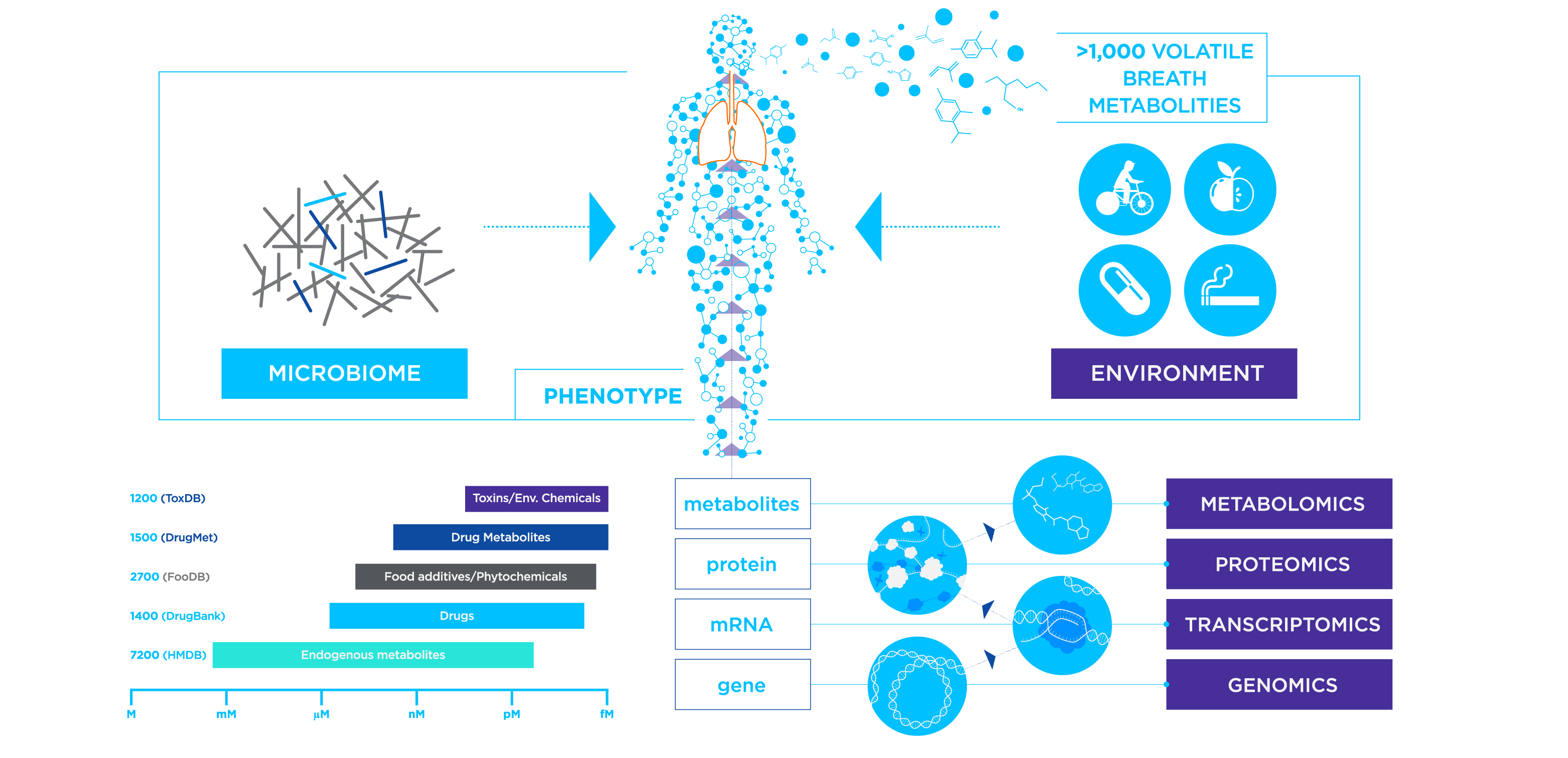
VOCs are carbon-based compounds that are gaseous at room temperature due to their chemical properties (such as high vapor pressure, and low boiling point). There are a few exceptions to this definition of VOCs, for example, although carbon dioxide has carbon and is gaseous at room temperature, it is not considered a VOC. An example of a VOC is the microbially-produced acetic acid, part of a volatile family of compounds known as the short-chain fatty acids. VOCs detectable in breath can originate from deeper within the body than the immediate respiratory tract, and can serve as biomarkers for a range of conditions.
The data gathered from Breath Biopsy analysis provides valuable insights into the patient phenotype that can be used for a range of applications across a broad range of disease areas, from biomarker discovery to early detection and precision medicine. Breath testing reports metabolic changes, often reflecting some of the earliest evidence of disease development, so is well suited to detecting disease progression, treatment responses, or relapse before other biomarker modalities.
We are currently working on developing our own point-of-care clinical tests using the Breath Biopsy platform, but also provide our capabilities and experience, in the form of products and services, to other companies and institutions to support their research and address critical clinical needs.
What can Breath Biopsy do for me?
Breath Biopsy has many distinct advantages, improving upon other breath collection techniques, leading to a robust and reliable performance across various applications.
Find clinically relevant, non-invasive biomarkers for respiratory and other diseases
Breath VOCs are a non-invasive source of data proximal to the lungs, forgoing the need for invasive biopsy procedures to acquire biomarker information for lung conditions. Sample collection is safe, pain-free and well tolerated by a variety of different patients, even those with breathing difficulties or reduced lung function.
However, Breath Biopsy can be used for far more than just respiratory disease. VOCs perfuse via the blood into the airways from all tissues in the body, meaning Breath Biopsy provides various insights on a wide range of disease areas including, gastroenterology, hepatology, and oncology. For example, our work on the early detection of chronic liver diseases.
Stratify patient phenotypes
Exhaled VOCs provide an orthogonal non-invasive dataset, complementing information from metabolomics, proteomics, and genomics. This can be used to support the characterization of novel phenotypes in complex diseases that could be targeted with drug treatments and to support clinical drug development.
Develop ways to sample patients at home or in a primary care setting
Once VOC biomarkers have been identified, Breath Biopsy sample collection could be performed in the patient’s home, or a local primary care site. This makes the approach well-suited for screening programs that could improve the early detection of conditions such as lung cancer, and for ongoing monitoring of disease progression and treatment responses.
As one example Owlstone Medical currently perform hydrogen/methane breath testing (e.g., for SIBO) by shipping collection kits to patients’ homes – patients take their breath samples and ship these back to us for analysis. Depending on the use-case and the biomarkers involved, it could even be possible to both collect and analyze breath samples rapidly within the patient’s home.
How can Owlstone Medical help you?
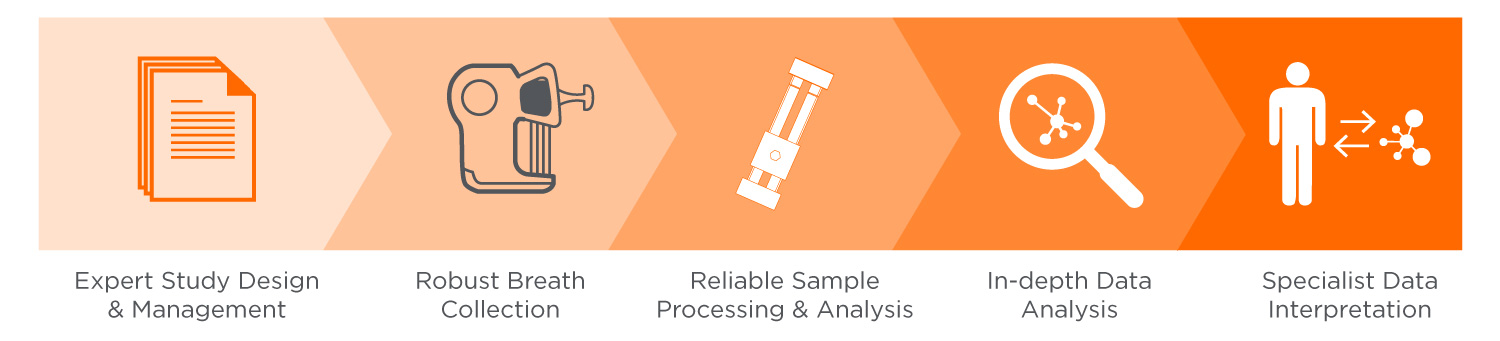
Our Breath Biopsy Services team is made up of individuals with world-leading breath analysis experience, with over 15 years of experience in VOC analysis and more than five years investigating breath as a source of clinically relevant biomarkers. Our team can provide specialist support in study planning and design, clinical trial management, statistical analysis of results, and biological interpretation of findings.
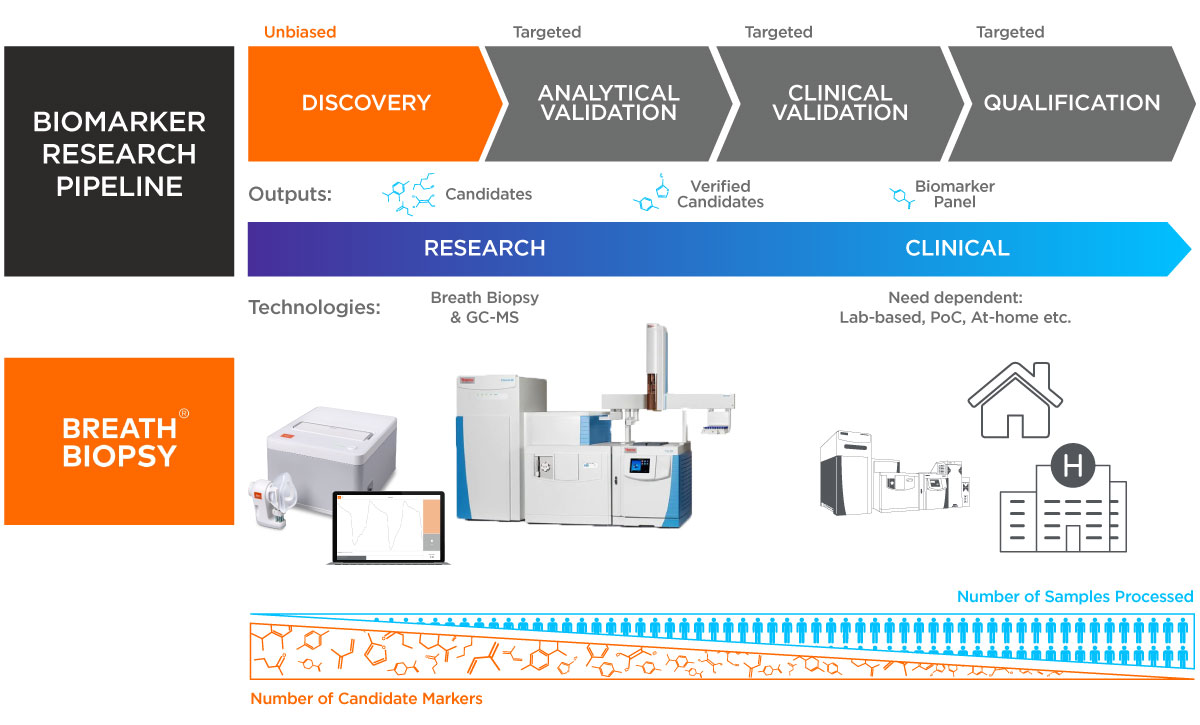
The starting point of breath biomarker development is our global biomarker discovery service, Breath Biopsy® OMNI. It is focused on identifying clinically translatable candidate biomarkers – i.e. ‘on-breath’ compounds derived from human subjects rather than the environment. To increase the chance of biomarker discovery, we have optimized OMNI to reliably detect hundreds of on-breath VOCs whilst reducing background noise.
Also critical to breath biomarker translation is the accurate chemical identification of candidate VOCs, such that results can be measured and validated on a wide range of analytical platforms. In OMNI, extensive internal standards maintain consistently high quality and help to facilitate subsequent biomarker quantitation and identification using our custom HRAM Breath Biopsy library.
Following the discovery process, we can also help translate results to the clinic, partnering with you to expedite the delivery of a qualified biomarker assay or breath test. Our work on FAIMS and other chemical sensors has provided us with extensive experience in developing and releasing sensor-based chemical detection systems for various industrial applications. We are therefore well placed to help with both the development and subsequent deployment of clinical tests.
For a complete overview of Breath Biopsy OMNI you can download our whitepaper. If you are interested in reliable VOC analysis and the potential for breath-based clinical tests, please contact us.
