Breath VOC Analysis as an Innovation to Fight Infectious Diseases: Malaria
Published on: 29 Apr 2024
Infectious disease is a huge burden on today’s society, causing millions of deaths every year. The impact of infectious disease has been particularly visible during recent epidemics such as the COVID-19 pandemic or the 2013–2016 Ebola virus disease epidemic in West Africa (1). More recently, we have developed vaccines and medicines to fight infections, yet diseases like Malaria and Tuberculosis continue to take lives worldwide. In low-income countries, 6 of the top 10 leading causes of death are communicable diseases (4). In the fight against infectious diseases, more research needs to be undertaken to develop new treatments and diagnostic tools to curb the spread of infection. Breath analysis offers opportunities to gain a greater mechanistic understanding of infectious disease, monitor its status and progression, as well as develop tests for early, rapid, and non-invasive diagnosis of disease. To support researchers interested in studying breath across different disease area, we have created the Breath Biopsy VOC Atlas®. This growing reference database already contains 80 malaria–associated VOCs. Malaria is a key example that will be the focus of this blog, but first, why use breath?
Why use breath?
Volatile organic compounds (VOCs) can originate from all around the body, and travel to the lungs via blood vessels to be excreted. VOCs diffuse into the air that is breathed in and can be detected in exhaled breath when breathed out. These molecules can be endogenous, meaning that they originate from internal metabolic processes ongoing in the body, for example, being produced by the immune system in response to a pathogen. VOCs can also be exogenous. Such external sources include dietary intake, or microbial metabolism (including the microbiome). The various points of origin allow for both the host response to disease and the pathogen’s mechanisms of action to be studied, improving the mechanistic understanding of disease. Infections that impact the respiratory system can be particularly relevant when utilizing breath analysis, as VOCs generated locally in the tissues of the respiratory tract can directly enter the breath, compared to more distal sampling mediums like blood or urine.
Despite extensive diagnostic developments in recent years, invasive tests are still being used to diagnose infectious diseases such as pulmonary tuberculosis (TB). Currently, rapid tests for TB include the skin Mantoux test and interferon-gamma release assays which are often unable to determine whether infection is active or latent (5). The accurate diagnosis of active TB infections therefore requires the use of invasive and expensive tests such as x-rays of the chest, mucus samples, and biopsies of infected tissue (4). These are time-consuming procedures that require specialist personnel to interpret the results, limiting their application in screening programs or for rapid, early diagnosis. Breath analysis could provide a simpler and less invasive approach for TB and other infectious disease diagnoses, particularly through the development of point-of-care (POC) devices to deliver rapid, accurate, and cheaper diagnoses.
Figure 1: Graphical representation of the Owlstone Medical handheld ReCIVA® Breath Sampler
Using EVOC® Probes
A cutting-edge approach to breath testing for infectious diseases is using exogenous VOC (EVOC) probes. EVOC probes are exogenous compounds administered to a patient to produce a detectable VOC product within the patient’s breath that is not normally found (or is normally found at much lower concentrations). This can greatly improve the reliability of biomarker detection and the accuracy of disease diagnosis. The EVOC approach is particularly applicable for infectious diseases as the unique biology of pathogens provides distinct metabolic pathways that can be targeted. Designing EVOC probes to evoke a reaction specific to a pathogen species could allow for the accurate and reliable diagnosis of infectious disease. This is similar to the approach used to diagnose H. pylori infections in the stomach, where a patient ingests 13C-urea and the presence of ammonia (as a specific product of H. pylori bacterial metabolism) in the breath in response.
Many studies have already begun to identify and characterize the VOCs associated with certain infectious diseases. Breath biomarkers for viral, bacterial, fungal, and parasitic diseases have all been identified, including for TB, invasive aspergillosis, and malaria infections (7–9). The case study below explores Malaria as a specific example of how breath analysis can be beneficial for research, diagnosis, and treatment of an infectious disease.
Breath VOC analysis as an innovative solution for malaria diagnosis
Malaria is a life-threatening infectious disease caused by the parasite plasmodium, which can infect humans through mosquito bites. Whilst often presenting with symptoms such as a high fever and headaches, more severe manifestations of the disease include severe fatigue, seizures, confusion, and difficulty breathing. Malaria is particularly life-threatening to young children, immuno-compromised individuals, and pregnant women, with 249 million cases and 608,000 deaths recorded in 2022 (10).
Figure 2: Mosquito on human skin.
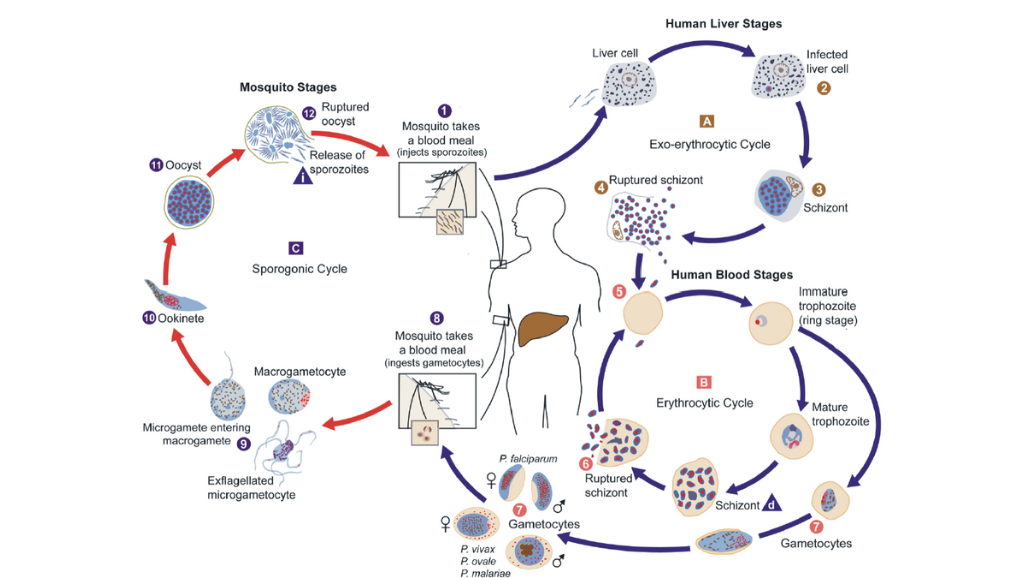
There are 5 different species of plasmodium parasite able to cause malaria in humans. Plasmodium falciparum is the most common cause of severe malaria, and is responsible for 90% of malaria mortality worldwide (11). In contrast, the second most common malaria-causing parasite plasmodium vivax causes fewer severe infections than falciparum. However, vivax has added complications for relapsing infections due to the liver stage of the complex lifecycle of infection where parasites can lie dormant, and therefore requires different methods of treatment (12).
Figure 3: Diagram of the plasmodium life cycle by Doerig and Grevelding 2015, (11).
These different species of plasmodium have distinct metabolisms, meaning that they may produce different VOC profiles. Detection of VOCs through breath analysis therefore has potential for the stratification of malaria infection. By being able to differentiate between different plasmodium species, clinicians may be better informed in deciding the best treatment options for patients.
Multiple VOCs indicative of malaria infection have already been identified. A 2018 paper by Schaber et al. identified alpha-pinene and 3-carene, compounds known to be mosquito attractants, at significantly higher levels on the breath of children with uncomplicated falciparum malaria (14). Furthermore, 6 other compounds also identified on the breath of these children, namely methyl undecane, dimethyl decane, trimethyl hexane, nonanal, isoprene, and tridecane, were used to diagnose malaria in the children with 83% accuracy. These six compounds can be found within our VOC ATLAS, a reference library that provides high-confidence identification of compounds for improved ability to develop VOC biomarkers for breath tests for clinical breath tests. With further characterization and validation of these VOCs, they could be utilised in the development of specific diagnostic tests for malaria using our Breath Biopsy® technology.
The current gold-standard technique for malaria diagnosis remains malarial microscopy, in which blood samples are examined under a microscope to visually identify parasites infecting the blood (13). This method of diagnosis requires specialist equipment, laboratory analysis, and trained staff, making it time-consuming, costly, and inaccessible to rural communities in malaria-endemic regions. Rapid antigen tests, however, provide in-field alternatives for diagnosis using blood samples to detect antigens of the parasite. Yet these tests are less reliable than microscopy as they are unable to detect lower pathogen loads or less common plasmodium species. This means that rapid tests are commonly followed up with the more reliable microscopy technique to confirm the results. Thus, there is a need to introduce a quick and effective point-of-care diagnosis method to reliably detect malaria in endemic settings and as demonstrated, VOC breath analysis provides an opportunity to develop a novel, non-invasive solution.
Dr. Audrey John from the Children’s Hospital of Philadelphia recently presented at the Breath Biopsy Conference 2024, on ‘Breath-based Diagnostics of Pediatric Malaria’. You can watch Dr. John’s talk on-demand, as well as the rest of the presentations from the conference here.
Breath Biopsy VOC Atlas®
In a recent literature review of 34 research papers on malaria, 158 VOCs were detected, 80 of which were added to the Breath Biopsy VOC Atlas®. The VOC Atlas is a catalog of confidently identified volatile organic compounds (VOCs) found in exhaled breath, providing insight and scientific context to identified compounds to enable the confident selection of candidate biomarkers for a variety of diseases.
The following table demonstrates VOCs with the potential to become malaria-specific biomarkers. Over half of these on-breath compounds are classified as terpenes, which are considered as mosquito attractants. Whether the altered terpene levels in humans with malaria resulted from the production of terpenes by the parasites requires further investigation. Regardless of the unknown mechanism, terpenes, in the context of malaria, are strong potential biomarkers for development.
Aldehydes are the chemical class with the second-highest counts in the malaria literature search. All the identified aldehydes are included in the VOC Atlas. Aldehydes are intermediate compounds of lipid peroxidation, which is associated with inflammation. While further research is needed to determine whether aldehyde levels in human breath are altered during parasite dormancy, their association with inflammation suggests that aldehydes could be potential markers for infection status in malaria.
Table 1. List of the top two chemical classes associated with malaria and compounds reported more than twice in literature review.
Chemical class |
Compound name |
Count of compound name in literature |
In Atlas |
On-breath in Atlas |
Terpenes |
α-Pinene | 9 | Yes | Yes |
| limonene | 7 | Yes | Yes | |
| β-pinene | 4 | Yes | Yes | |
| 3-carene | 3 | Yes | Yes | |
| terpinolene | 2 | Yes | Yes | |
| m-cymene | 2 | Yes | Yes | |
Aldehydes |
nonanal | 14 | Yes | No |
| octanal | 8 | Yes | No | |
| decanal | 7 | Yes | No | |
| hexanal | 5 | Yes | No | |
| 3-methylbutanal | 3 | Yes | No | |
| 2-methyl-2-butanal | 2 | Yes | No | |
| phenylacetaldehyde | 2 | Yes | No | |
| 2-methylbutanal | 2 | Yes | No | |
| heptanal | 2 | Yes | No | |
| (E)-2-decenal | 2 | Yes | No | |
| benzaldehyde | 2 | Yes | No |
The VOC Atlas also includes a subset of VOCs detectable in a heterogeneous human cohort, which provide a useful baseline to streamline breath biomarker discovery and validation, for example, to identify VOC targets that are likely to be impacted by the disease process of interest. If you would like early access to the Atlas, you can contact us directly or sign up here.
References:
Baker RE, Mahmud AS, Miller IF, Rajeev M, Rasambainarivo F, Rice BL, et al. Infectious disease in an era of global change. Nat Rev Microbiol. 2022 Apr;20(4):193–205. https://doi.org/10.1038/s41579-021-00639-z
The top 10 causes of death [Internet]. [cited 2024 Apr 14]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
Jin J. Screening for Latent Tuberculosis. JAMA. 2023 May 2;329(17):1526. doi: 10.1001/jama.2023.6495
nhs.uk [Internet]. 2017 [cited 2024 Mar 11]. Tuberculosis (TB). Available from: https://www.nhs.uk/conditions/tuberculosis-tb/
Ibrahim W, Cordell RL, Wilde MJ, Richardson M, Carr L, Dasi ASD, et al. Diagnosis of COVID-19 by exhaled breath analysis using gas chromatography–mass spectrometry. ERJ Open Res [Internet]. 2021 Jul 1 [cited 2024 Mar 11];7(3). Available from: https://openres.ersjournals.com/content/7/3/00139-2021. DOI: 10.1183/23120541.00139-2021
6. Koo S, Thomas HR, Daniels SD, Lynch RC, Fortier SM, Shea MM, et al. A Breath Fungal Secondary Metabolite Signature to Diagnose Invasive Aspergillosis. Clin Infect Dis. 2014 Dec 15;59(12):1733–40. DOI: 10.1093/cid/ciu725
Phillips M, Basa-Dalay V, Bothamley G, Cataneo RN, Lam PK, Natividad MPR, et al. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis. 2010 Mar 1;90(2):145–51. DOI: 10.1016/j.tube.2010.01.003
Fact sheet about malaria [Internet]. [cited 2024 Mar 5]. Available from: https://www.who.int/news-room/fact-sheets/detail/malaria
Zekar L, Sharman T. Plasmodium falciparum Malaria. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 [cited 2024 Mar 10]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK555962/
10. Chu CS, White NJ. Management of relapsing Plasmodium vivax malaria. Expert Rev Anti Infect Ther. 2016 Oct 2;14(10):885–900. DOI: 10.1080/14787210.2016.1220304
11. Doerig C, Grevelding CG. Targeting kinases in Plasmodium and Schistosoma: Same goals, different challenges. Biochim Biophys Acta BBA – Proteins Proteomics. 2015 Oct 1;1854(10, Part B):1637–43. DOI: 10.1016/j.bbapap.2015.03.002
12. Schaber CL, Katta N, Bollinger LB, Mwale M, Mlotha-Mitole R, Trehan I, et al. Breathprinting Reveals Malaria-Associated Biomarkers and Mosquito Attractants. J Infect Dis. 2018 May 15;217(10):1553–60. DOI: 10.1093/infdis/jiy072
13. Tangpukdee N, Duangdee C, Wilairatana P, Krudsood S. Malaria Diagnosis: A Brief Review. Korean J Parasitol. 2009 Jun;47(2):93–102. doi: 10.3347/kjp.2009.47.2.93
Quick Start Guide: Everything you need to know about how breath analysis can be used in infectious disease research