Breath VOC biomarkers for infectious diseases
Research has indicated that VOCs in breath are associated with infectious diseases, showing promise as non-invasive biomarkers.
| Disease Area: Infectious Diseases
Aim: To better understand the potential of breath analysis in the early diagnosis of infectious diseases. Sample Medium: Breath Approach: Literature Search Summary:
|
Breath analysis relies on the idea that the volatile organic compound (VOC) profile of exhaled breath is altered as a response to underlying pathological states and that these VOCs can be detected and measured to be used for disease diagnosis and monitoring. Exhaled VOCs reflect not only the metabolic processes occurring in the respiratory tract but also metabolic activity occurring in distal parts of the body. This is because circulating VOCs in the blood may diffuse into the alveolar space for exhalation.
The non-invasive nature of breath analysis makes it attractive for disease diagnosis and monitoring as repeat measurements can be taken with minimal discomfort to the participating patients. VOCs in breath can originate from the environment, endogenous host metabolism, and the metabolic activity of microbes in the body, including pathogens and the microbiome in the gut and airways. Endogenous VOCs have the potential to provide a window into the physiological state of an individual, and microbial VOCs could assist in the identification of pathogens.
An infectious disease can be defined as an illness due to a pathogen or its toxic product, which arises through transmission from an infected person, infected animal, or contaminated object to a host. Infectious diseases are responsible for a huge global burden of disease that impacts public health systems and economies worldwide, especially affecting vulnerable populations. Lower respiratory tract infections, diarrheal diseases, malaria, and tuberculosis (TB) are among the top causes of overall global mortality (1).
Research has indicated that VOCs in breath are associated with infectious diseases, showing promise as non-invasive biomarkers. Pathogenic microbes can alter the VOCs in breath either through altered metabolic pathways in the host such as the immune response, inflammation to the affected tissues, or with certain pathogens like bacteria and fungi, their own endogenous metabolic processes.
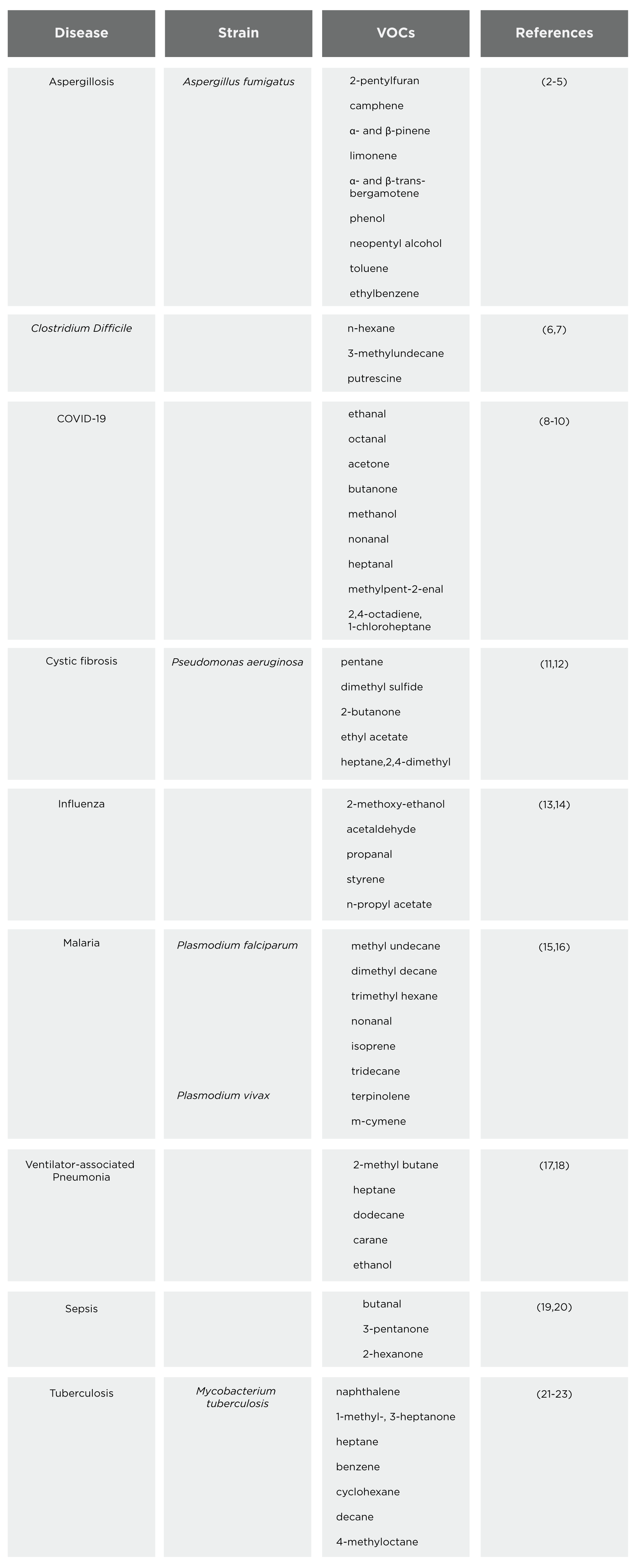
Some infectious diseases predominantly affect the lungs and other tissues in the respiratory tract such as the nose and throat, and as breath is in direct contact with these tissues, breath analysis can provide a direct insight into the disease as opposed to other more indirect sampling mediums such as blood or urine. We completed a literature review to summarize VOC biomarkers for infectious diseases, Table 1 highlights our findings.
VOCs in breath are associated with infectious disease
This table presents a comprehensive summary of VOCs associated with various infectious diseases, offering insights into potential biomarkers for diagnosis and monitoring. Diseases covered include aspergillosis, C. diff infection, COVID-19, cystic fibrosis, influenza, malaria, pneumonia, sepsis, and tuberculosis. The compounds identified by these studies require further investigation to discover the disease-related pathophysiology that leads to their changing abundance in the breath.
Out of the 56 compounds identified in this literature search, 25 of them were contained within our Breath Biopsy® VOC Atlas. Our VOC Atlas contains VOCs that have been carefully quantified for their presence in the breath (‘on-breath’) of a population of heterogeneous volunteers without any specific pathophysiology, and all VOCs have undergone rigorous validation to confirm their identities. There are two groups of compounds included in the Atlas – ‘on-breath’ and ‘off-breath’. On-breath compounds are thought to be associated with normal variation in the population, whereas off-breath compounds in the VOC Atlas are compounds that are not regularly observed in healthy individuals.
This means that off-breath compounds may only be detectable in the breath of those with disease and not in the breath of a healthy person, and therefore are promising to investigate further as potential breath biomarkers of disease. In this literature search, there were a total of 9 on-breath compounds and 16 off-breath compounds. Aldehydes such as octanal and heptanal (found in the breath of COVID-19 patients) are off-breath compounds that can be formed endogenously by lipid peroxidation, which could suggest that lipid peroxidation may be a source of the altered VOC signature seen in the breath of patients with infectious diseases.
Inflammation of the airways is a common characteristic of many infectious diseases, generating oxidative stress in the tissues of the respiratory tract. Oxidative stress causes the release of compounds, including alkanes. Decane (found in the breath of tuberculosis patients), heptane (found in the breath of pneumonia patients), and pentane (found in the breath of cystic fibrosis patients) are examples of off-breath alkanes found in this literature search that could be due to oxidative stress. However, it is important to note that whilst off-breath compounds do have the potential of being off-breath in a healthy population and on-breath in disease (making them ideal biomarkers), there is also clinical potential in on-breath compounds that are significantly increased/decreased in disease.
To progress these biomarkers towards validation, and eventually translation into further breath tests for infectious disease in clinical use, more work needs to be done to link the characteristic changing levels of these compounds in the breath in infectious disease, and the underlying mechanisms responsible. We can incorporate the use of breath analysis into your infectious disease research as part of our Breath Biopsy OMNI® service to enhance and fast-track breath biomarker identification and validation. To find out more information about breath biomarkers for infectious disease, and other potential research studies involving the use of VOCs, please do not hesitate to contact us.
References.
- van Seventer JM, Hochberg NS. Principles of Infectious Diseases: Transmission, Diagnosis, Prevention, and Control. Int Encycl Public Health. 2017;22–39.
- Chambers ST, Syhre M, Murdoch DR, McCartin F, Epton MJ. Detection of 2-Pentylfuran in the breath of patients with Aspergillus fumigatus. Med Mycol. 2009 Aug 1;47(5):468–76.
- Syhre M, Scotter JM, Chambers ST. Investigation into the production of 2-Pentylfuran by Aspergillus fumigatus and other respiratory pathogens in vitro and human breath samples. Med Mycol. 2008 May 1;46(3):209–15.
- Koo S, Thomas HR, Daniels SD, Lynch RC, Fortier SM, Shea MM, et al. A Breath Fungal Secondary Metabolite Signature to Diagnose Invasive Aspergillosis. Clin Infect Dis. 2014 Dec 15;59(12):1733–40.
- Li ZT, Zeng PY, Chen ZM, Guan WJ, Wang T, Lin Y, et al. Exhaled Volatile Organic Compounds for Identifying Patients With Chronic Pulmonary Aspergillosis. Front Med. 2021; Vol 8.
- John TM, Shrestha NK, Hasan L, Pappan K, Birch O, Grove D, et al. Detection of Clostridioides difficile infection by assessment of exhaled breath volatile organic compounds. J Breath Res. 2024 Mar;18(2):026011.
- John TM, Shrestha NK, Procop GW, Grove D, Jr SML, Jacob CN, et al. Diagnosis of Clostridioides difficile infection by analysis of volatile organic compounds in breath, plasma, and stool: A cross-sectional proof-of-concept study. PLOS ONE. 2021 Aug 18;16(8):e0256259.
- Ibrahim W, Cordell RL, Wilde MJ, Richardson M, Carr L, Dasi ASD, et al. Diagnosis of COVID-19 by exhaled breath analysis using gas chromatography–mass spectrometry. ERJ Open Res. 00139-2021.
- Berna AZ, Akaho EH, Harris RM, Congdon M, Korn E, Neher S, et al. Reproducible Breath Metabolite Changes in Children with SARS-CoV-2 Infection. ACS Infect Dis. 2021 Sep 10;7(9):2596–603.
- Grassin-Delyle S, Roquencourt C, Moine P, Saffroy G, Carn S, Heming N, et al. Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study. eBioMedicine. 2020; Vol 63, 103154.
- Barker M, Hengst M, Schmid J, Buers HJ, Mittermaier B, Klemp D, et al. Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur Respir J. 2006 May 1;27(5):929–36.
- Kos R, Brinkman P, Neerincx AH, Paff T, Gerritsen MG, Lammers A, et al. Targeted exhaled breath analysis for detection of Pseudomonas aeruginosa in cystic fibrosis patients. J Cyst Fibros. 2022 Jan 1;21(1):e28–34.
- Aksenov AA, Sandrock CE, Zhao W, Sankaran S, Schivo M, Harper R, et al. Cellular Scent of Influenza Virus Infection. Chembiochem Eur J Chem Biol. 2014 May 5;15(7):1040–8.
- Traxler S, Bischoff AC, Saß R, Trefz P, Gierschner P, Brock B, et al. VOC breath profile in spontaneously breathing awake swine during Influenza A infection. Sci Rep. 2018 Oct 5;8(1):14857.
- Schaber CL, Katta N, Bollinger LB, Mwale M, Mlotha-Mitole R, Trehan I, et al. Breathprinting Reveals Malaria-Associated Biomarkers and Mosquito Attractants. J Infect Dis. 2018 Apr 23;217(10):1553–60.
- Chai HC, Chua KH. The Potential Use of Volatile Biomarkers for Malaria Diagnosis. Diagnostics. 2021 Nov 30;11(12):2244.
- Oort P van, Bruin S de, Weda H, Knobel H, Schultz M, Bos L. Exhaled breath metabolomics for the diagnosis of pneumonia in intubated and mechanically ventilated ICU–patients. Eur Respir J 2017; 50: OA4653
- Schnabel R, Fijten R, Smolinska A, Dallinga J, Boumans ML, Stobberingh E, et al. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Sci Rep. 2015 Nov 26;5(1):17179.
- Fink T, Wolf A, Maurer F, Albrecht FW, Heim N, Wolf B, et al. Volatile Organic Compounds during Inflammation and Sepsis in Rats: A Potential Breath Test Using Ion-mobility Spectrometry. Anesthesiology. 2015 Jan 1;122(1):117–26.
- Kauppi AM, Edin A, Ziegler I, Mölling P, Sjöstedt A, Gylfe Å, et al. Metabolites in Blood for Prediction of Bacteremic Sepsis in the Emergency Room. PLOS ONE. 2016 Jan 22;11(1):e0147670.
- Phillips M, Cataneo RN, Condos R, Ring Erickson GA, Greenberg J, La Bombardi V, et al. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis. 2007 Jan 1;87(1):44–52.
- Phillips M, Basa-Dalay V, Bothamley G, Cataneo RN, Lam PK, Natividad MPR, et al. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis. 2010 Mar 1;90(2):145–51.
- Bobak CA, Kang L, Workman L, Bateman L, Khan MS, Prins M, et al. Breath can discriminate tuberculosis from other lower respiratory illness in children. Sci Rep. 2021 Feb 1;11(1):2704.
Quick Start Guide: Everything you need to know about how breath analysis can be used in infectious disease research