Breath analysis for diagnosis of Pulmonary Arterial Hypertension
PAH is often diagnosed late. Early detection is critical to improve clinical outcomes.
| Publication information: Morad K. Nakhleh et al., Volatolomics of breath as an emerging frontier in pulmonary arterial hypertension, Eur Respir J. 2017 49: 1601897. DOI: 10.1183/13993003.01897-2016
Disease Area: Pulmonary Arterial Hypertension Application: Early detection Sample medium: Breath Analysis approach: Gas Sensors, GC-MS Summary:
|
Pulmonary Arterial Hypertension (PAH) is a progressive disorder where arteries in the lungs narrow, raising blood pressure and forcing the heart to work harder to pump blood throughout the body. Around 15-60 people in every million are affected and if left untreated it results in death within, on average, 2.8 years. While existing treatments can manage PAH it remains incurable – with lung transplantation required in severe cases.
Currently PAH is most frequently diagnosed at a late stage, when therapeutic interventions must be more drastic and mortality rates are high. Right heart catheterization provides a definitive diagnosis but, as an extremely invasive procedure, is not suitable for use in screening programs. The early phases of PAH are often considered ‘clinically silent’ – while there are some obvious symptoms they are non-specific, further complicating early diagnosis.
Other tools for assessing PAH do exist (such as Doppler echocardiography, and lung function and exercise tests) but their diagnostic value has not been conclusively established. If reliable and non-invasive biomarkers could be established for PAH this would make screening high-risk populations possible, increasing the rate of earlier diagnoses – when therapeutic interventions are cheaper and patient outcomes are better.
Volatile organic compounds (VOCs) are gaseous molecules that can be sampled quickly and non-invasively from breath with Breath Biopsy®. Some of the VOCs on exhaled breath are produced endogenously within the body by physiological processes. With the right breath collection and analytical equipment (such as the Breath Biopsy Collection Station and thermal desorption gas chromatography mass spectrometry) these VOCs can be detected on breath, at even very low concentrations (parts per trillion).
When physiological processes are altered as a result of disease, the production of corresponding VOCs should alter too – which will likely be reflected in the VOC profile of exhaled breath. Indeed, such changes have already been noted in many studies that have looked at using breath as a diagnostic tool for other respiratory diseases.
There might be changes in the concentrations of VOCs on the exhaled breath of a person with developing PAH, as a consequence of the ‘process of remodeling that occurs [in their lungs] at the level of the arterioles’. This process is thought to result in local hypoxia, leading to anaerobic metabolism by the cells and significant elevated levels of VOCs such as ketones and alcohols being produced, that would be detectable on exhaled breath. Or, it could lead to raised metabolism of cholesterol, which would be detectable on breath through increased levels of isoprene. Additionally, inflammation, another common symptom of PAH, could cause an increase in reactive oxygen species (ROS), that through peroxidation of lipids and fatty acids might in turn drive up certain alkanes on exhaled breath. Beyond these stated examples, there are likely to be many other metabolic changes that are related to PAH and have a corresponding affect on breath VOCs.
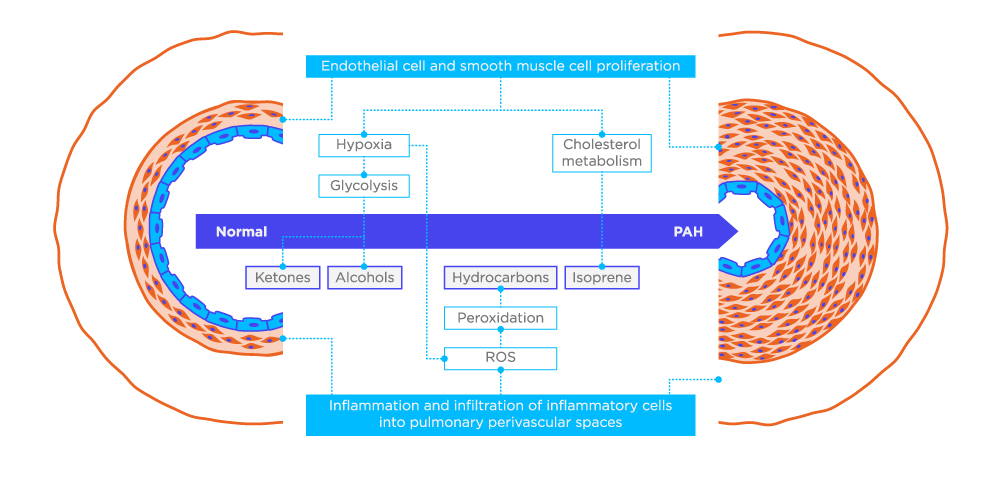
Figure 1. Hypothetical schema of possible VOC sources in PAH in a remodelled artery.
Methods
The study conducted by Nakhleh et al. used an array of nonselective gas sensors to analyze exhaled breath samples from 22 PAH patients and 23 healthy controls. The receiver operating characteristic-based classifier they created using this data was able to differentiate between groups with an accuracy of 92%. Further, the gas sensors were able to differentiate heritable PAH (n=7) patients from idiopathic (I)PAH patients (n=15), with an accuracy of 87%. The gas sensors also seemed capable of classifying the relative severity of cases, with 91% accuracy (according to the New York Heart Association classification).
Gas sensors, like eNoses, produce ‘breathprints’ profiling a person’s whole breath. They can be a cheap and easy way to compare similar profiles across groups, but they cannot be used to identify individual VOCs, which makes it hard to relate any observed differences back to underlying biology. For this reason, alongside their work with gas sensors, Nakhleh et al. also conducted quantitative analysis using gas chromatography mass spectrometry (GC-MS). This analytical work found significant differences in the concentrations of 10 VOCs which had previously been associated with PAH.1
Suggested further work
The cohort in Nakhleh et al’s study was small, so significant validation work, with an independent cohort is necessary. However, this study, and others like it, underlined the potential for breath to be used as part of a diagnostic strategy for the treatment of PAH. Nakhleh et al. suggest two different ways in which further investigation of breath biomarkers could be undertaken.
Firstly, it could be possible to develop a non-invasive breath test for the earlier diagnosis of PAH. ‘Arterial remodelling in the very early stages of PAH involves several molecular and cellular pathways that could affect the specific spectra of VOCs. This silent or vague phase can last up to 4 years before a formal diagnosis is made.’ To achieve this aim identifying and targeting of these VOCs should be a priority. This could be possible through ongoing monitoring, including regular breath sampling, of subjects at high-risk of developing PAH. If screening of high-risk patients is deemed impractical for a disease with such low incidence, an alternative would be to instead test all help-seekers reporting shortness of breath. The second proposed route to identify relevant VOCs for a breath test is through in vitro work, or even ex vivo analysis of tissue samples, (which it might be possible to acquire after PAH patients have received lung transplants).
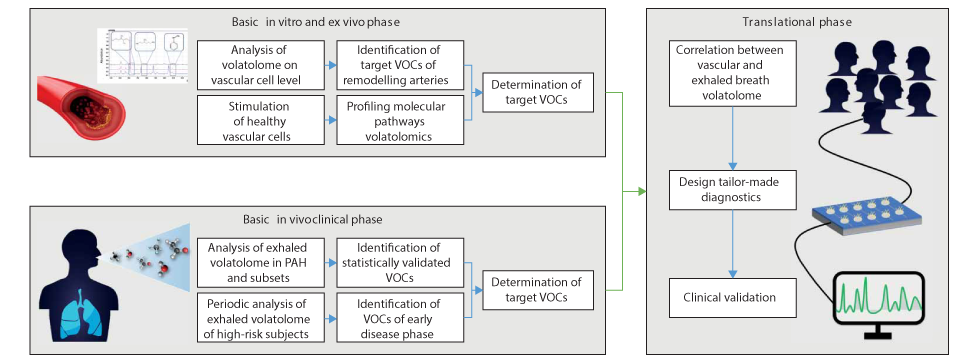
Figure 2. Schematic diagram of the suggested “bottom–top” experimental flowchart towards designated PAH diagnostics, based on exhaled volatolome analysis.
Nakhleh et al. conclude by restating that as long as right heart catheterization remains the gold-standard diagnostic method, PAH will continue to be diagnosed late – ‘negatively affecting clinical outcomes and survival rates’. The untapped potential of exhaled breath as a source of biomarkers could provide the solution. With further research it may be possible to determine the exhaled VOCs linked with the very early disease stages of PAH and then develop a non-invasive test that may even be suitable for widespread screening.
At Owlstone Medical, working with Morad Nakhleh and many other leading breath researchers, we have developed Breath Biopsy OMNI, the most advanced solution for reliable global breath VOC analysis. We are experts at running clinical trials with the best chance of identifying breath biomarkers and we also have experience using in vitro analysis to identify potential breath biomarkers of disease.
READ THE BREATH BIOPSY OMNI WHITEPAPER WATCH OUR IN VITRO WEBINAR
If you’re interested in investigating breath-based biomarkers for PAH, or another disease, we can help.
Additional references
- Nakhleh et al. Breath volatolome for the detection of pulmonary arterial hypertension Am J Crit Care Med (2016) 193: A7354. Meeting abstract online.



