Identifying Asthma and assessing control using Breath Biopsy
VOC biomarkers have potential for precision medicine and predicting exacerbations
| Publication information: Owlstone Medical internal data – Asthma white paper, LuCID study
Disease Area: Asthma Application: Precsion Medicine Sample medium: Breath Products: ReCIVA® Breath Sampler Analysis approach: GC-MS Summary:
|
Asthma is a chronic inflammatory condition affecting close to 350 million people worldwide1. Although all asthma patients experience a common set of symptoms, the underlying disease mechanisms vary between patients and this impacts treatment. Understanding the phenotypes responsible for asthma in each patient is key to improving treatment.
There is now a considerable move towards precision medicine in asthma, as highlighted by Dr Stephen Fowler at the 2018 Breath Biopsy Conference and by the Lancet Respiratory Asthma Task Force and others2,3.
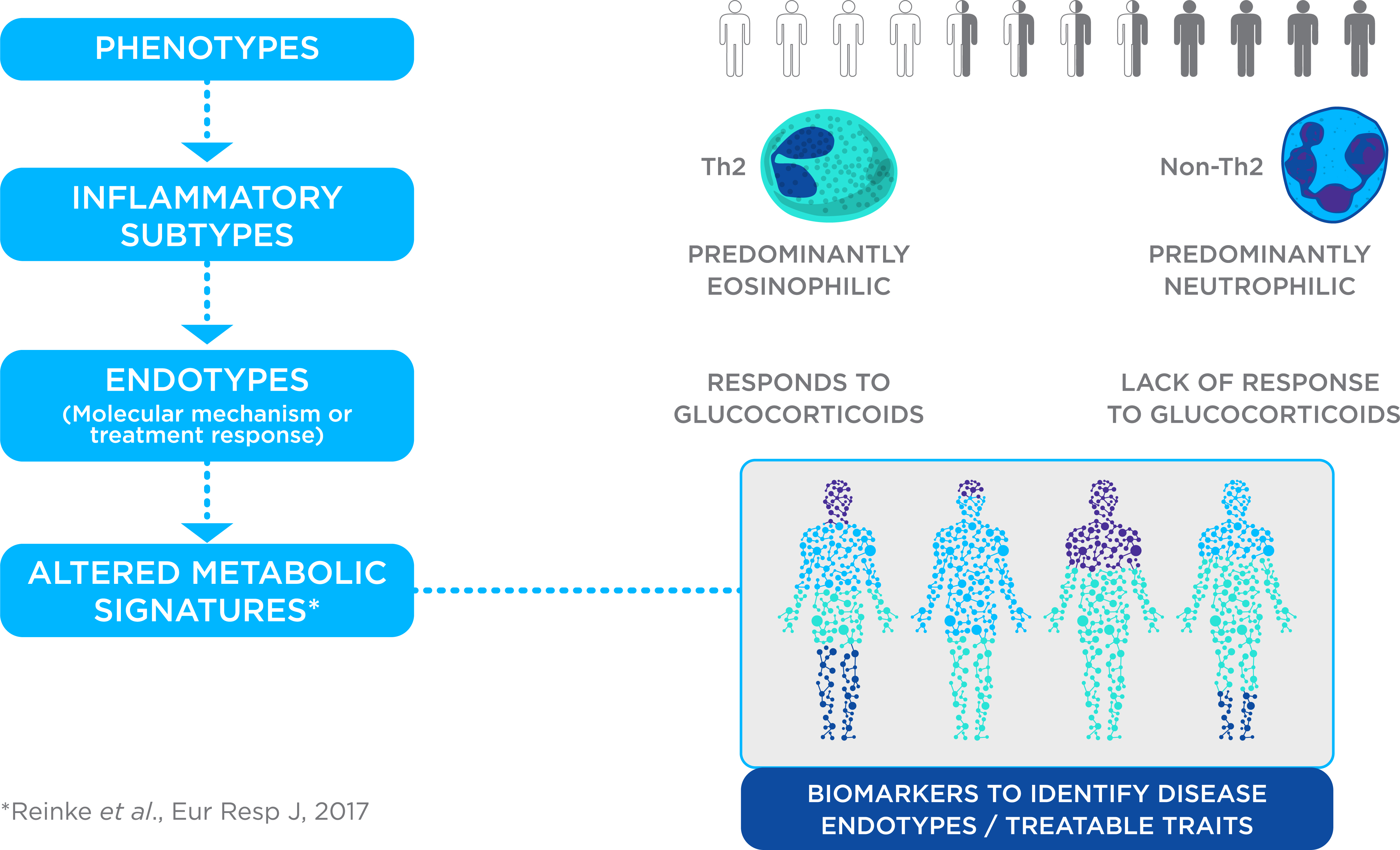
Figure 1. Precision Medicine for Asthma. Asthma management is challenging due to multiple phenotypes, inflammatory subtypes and endotypes with different underlying molecular mechanisms and responses to treatment. Breath testing is being explored as a route to discover biomarkers to identify disease endotypes or treatable traits, enabling a precision medicine approach to asthma treatment.
Currently treatment focuses on symptom management, and finding the right treatment can be a lengthy process of trial and error. A reliable test for different asthma phenotypes could improve the speed and efficiency of diagnosis and enable more widespread use of precision medicine. Research examining VOCs as a tool for precision medicine in adult asthma has been reviewed previously4,5.
Recent years have seen the approval of biological drugs such as XOLAIR®, NUCALA®, CINQAIR®, FASENRA®, and DUPIXENT® targeted at specific pathways relevant to inflammatory subtypes, yet their approval for clinical use has been delayed due to the high cost of the treatment, combined with the difficulty of identifying patients with the correct asthma phenotype who would benefit from the drug.
Studies have demonstrated that volatile organic compounds (VOCs) in exhaled breath can outperform existing measures, such as fractional exhaled nitric oxide (FENO) and lung function tests, as biomarkers when discriminating between asthmatics and healthy controls6. Van der Schee et al. found that VOCs in breath are able to predict responsiveness to steroid treatment in steroid-naïve, mild to moderate asthma patients7. With an area under the curve of 0.88, VOCs outperformed both FENO and measurements of eosinophil cells from sputum samples.
Discrimination of Asthmatic vs. Non-Asthmatic Patients with Breath Biopsy®
We’ve examined the ability of VOCs analyzed using Breath Biopsy to discriminate between asthmatic and non-asthmatic patients (Figure 2). As you can see, even in a heterogeneous clinical trial population, which included individuals with a wide variety of pulmonary conditions, the VOC profile discriminates well between patients with and without an asthma diagnosis.
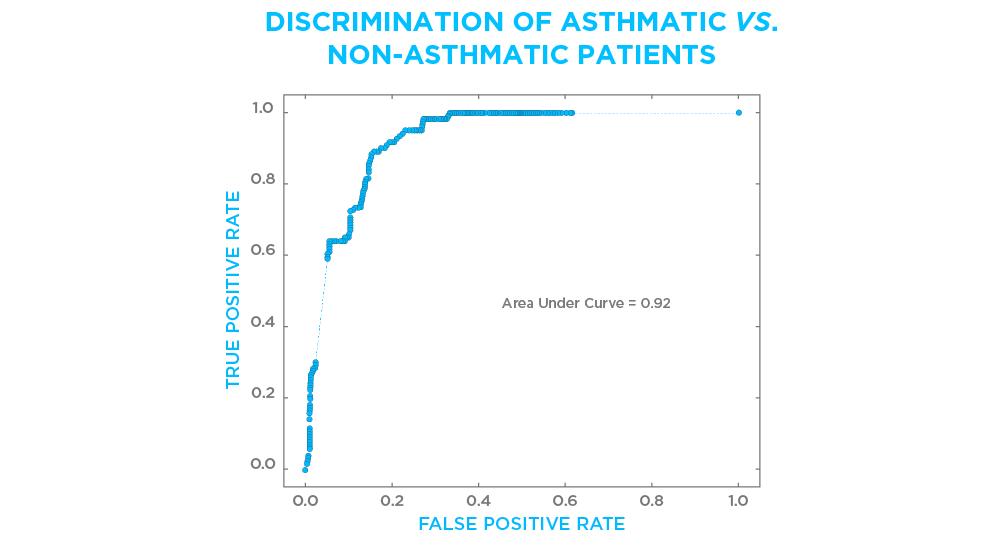
Figure 2. Breath VOCs analyzed using Breath Biopsy were used to build a classifier which could discriminate between patients with or without a diagnosis of asthma. The Receiver Operator Characteristics (ROC) curve shows an area under the curve of 0.92. The ROC evaluates the ability of a test to discriminate between states with 1.0 indicating a perfect test. The patient population included individuals with a wide variety of pulmonary conditions, participating in a larger clinical trial.
Breath Biopsy for Distinguishing Controlled vs. Uncontrolled Asthma
Reliable biomarkers to identify patients with uncontrolled asthma, or to predict exacerbations, would inform treatment decisions and facilitate disease management. The profile of VOCs in exhaled breath can be used to distinguish between patients with controlled and uncontrolled asthma (Figure 3), which is an important aspect of a patient’s asthma phenotype.
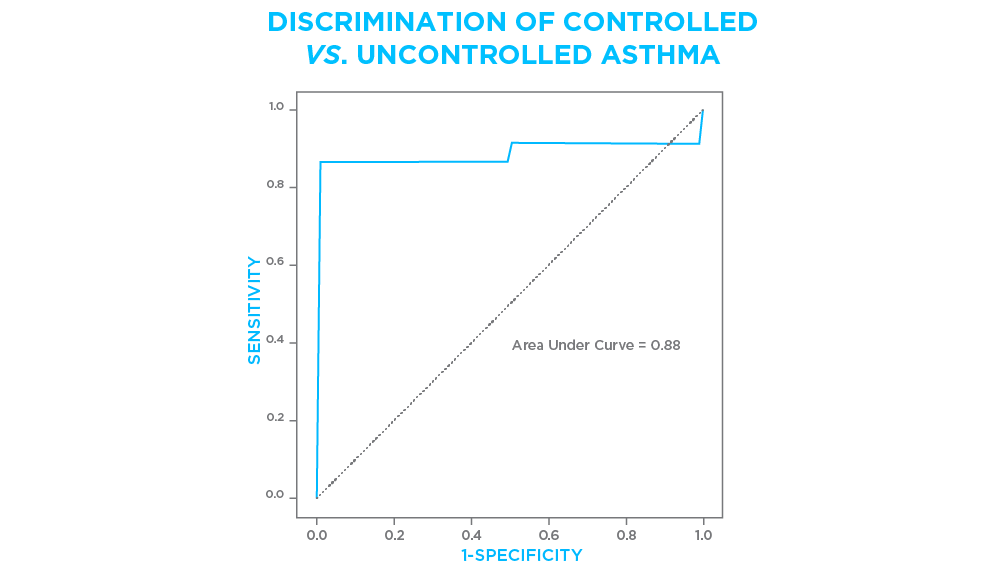
Figure 3. Breath VOCs analyzed using Breath were used to build a classifier which could identify individuals with self-reported uncontrolled asthma with 85% sensitivity and 98% specificity. ROC curve shows an area under the curve of 0.88. A subpopulation of individuals participating in a larger clinical trial were included in this analysis.
It has also been reported that breath VOCs analysed using our FAIMS technology can potentially predict loss of control in asthma8. In this study the Lonestar® VOC Analyzer, was shown to outperform sensor array type eNoses and gas chromatography-mass spectrometry (GC-MS).
Summary
References
- The Global Asthma Report, The Global Asthma Network (2018). http://www.globalasthmareport.org/index.html
- Pavord et al., After asthma: redefining airways diseases, The Lancet, (2017). DOI: 10.1016/S0140-6736(17)30879-6
- Chung, Personalised medicine in asthma: time for action, Eur. Resp. Rev., 26, (2017). DOI: 10.1183/16000617.0064-2017
- Azim et al., Exhaled volatile organic compounds in adult asthma: a systematic review, Eur. Resp. J., 54 (2019). DOI: 10.1183/13993003.00056-2019
- Brinkman et al. Exhaled volatile organic compounds as markers for medication use in asthma, Eur. Resp. J. 54 (2019). DOI: 10.1183/13993003.00544-2019
- Pité et al., Metabolomics in asthma: where do we stand?, Curr. Opin. Pulm. Med. (2017). DOI: 10.1097/MCP.0000000000000437
- van der Schee et al., Predicting steroid responsiveness in patients with asthma using exhaled breath profiling, Clin. Exp. Allergy. 43 (2013) 1217–1225. DOI: 10.1111/cea.12147
- Montuschi et al., Diagnostic performance of an electronic nose, fractional exhaled nitric oxide, and lung function testing in asthma, Chest. 137 (2010) 790–796. DOI: 10.1378/chest.09-1836
- The Global Asthma Report, The Global Asthma Network (2018). http://www.globalasthmareport.org/index.html
- Asthma and Allergy Foundation of America – Asthma Facts https://www.aafa.org/asthma-facts/
- Schleich et al., Exhaled Volatile Organic Compounds are Able to Discriminate between Neutrophilic and Eosinophilic Asthma, Am J Respir Crit Care Med, (2019). doi.org/10.1164/rccm.201811-2210OC
- Pavord et al., After asthma: redefining airways diseases, The Lancet, (2017). dx.doi.org/10.1016/S0140-6736(17)30879-6
- Chung, Personalised medicine in asthma: time for action, Eur. Resp. Rev., 26, (2017). dx.doi.org/10.1183/16000617.0064-2017
- Azim et al., Exhaled volatile organic compounds in adult asthma: a systematic review, Eur. Resp. J., 54 (2019). dx.doi.org/10.1183/13993003.00056-2019
- Brinkman et al. Exhaled volatile organic compounds as markers for medication use in asthma, Eur. Resp. J. 54 (2019). dx.doi.org/10.1183/13993003.00544-2019
- Reinke et al., Metabolomics analysis identifies different metabotypes of asthma severity, Eur. Resp. J., 49, (2017). dx.doi.org/10.1183/13993003.01740-2016
- Pité et al., Metabolomics in asthma: where do we stand?, Curr. Opin. Pulm. Med. (2017). dx.doi.org/10.1097/MCP.0000000000000437
- Ibrahim et al., Non-invasive phenotyping using exhaled volatile organic compounds in asthma., Thorax, 66 (2011) 804–809. dx.doi.org/10.1136/thx.2010.156695
- Montuschi et al., Diagnostic performance of an electronic nose, fractional exhaled nitric oxide, and lung function testing in asthma, Chest. 137 (2010) 790–796. dx.doi.org/10.1378/chest.09-1836
- van der Schee et al., Predicting steroid responsiveness in patients with asthma using exhaled breath profiling, Clin. Exp. Allergy. 43 (2013) 1217–1225. dx.doi.org/10.1111/cea.12147
- Brinkman et al., Exhaled volatie organic compounds as markers for medication use in asthma. Eur. Respir. J. (2019) 54 (4) doi.org/10.1183/13993003.00544-2019
- Santini et al., Discrimination between oral corticosteroid-treated and oral corticosteroid-non-treated severe asthma patients by an electronic nose platform, Eur. Respir. J. 44 (2014). erj.ersjournals.com/content/44/Suppl_58/P2054
- Santini et al., Breathomics can discriminate between anti IgE-treated and non-treated severe asthma adults, Eur. Respir. J. 46 (2015). http://erj.ersjournals.com/content/46/suppl_59/OA1463
- Dallinga et al., Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children, Clin. Exp. Allergy., 40 (2010). 68-76 40:1 68-76 doi.org/10.1111/j.1365-2222.2009.03343.x
- Smolinska et al., Profiling of volatile organic compounds in exhaled breath as a strategy to find early predictive signatures of asthma in children, PLoS One (2014). doi.org/10.1371/journal.pone.0095668
- Neerincx et al., Breathomics from exhaled volatile organic compounds in pediatric asthma, Pediatric Pul. 52 (12) 1616-1627. (2017). doi.org/10.1002/ppul.23785